Maximum life span
Maximum life span (or, for humans, maximum reported age at death) is a measure of the maximum amount of time one or more members of a population have been observed to survive between birth and death. The term can also denote an estimate of the maximum amount of time that a member of a given species could survive between birth and death, provided circumstances that are optimal to that member's longevity.
Most living species have at least one upper limit on the number of times the cells of a member can divide. This is called the Hayflick limit, although number of cell divisions does not strictly control lifespan.
Definition
In animal studies, maximum span is often taken to be the mean life span of the most long-lived 10% of a given cohort. By another definition, however, maximum life span corresponds to the age at which the oldest known member of a species or experimental group has died. Calculation of the maximum life span in the latter sense depends upon initial sample size.[1]
Maximum life span contrasts with mean life span (average life span, life expectancy), and longevity. Mean life span varies with susceptibility to disease, accident, suicide and homicide, whereas maximum life span is determined by "rate of aging".[2] Longevity refers only to the characteristics of the especially long lived members of a population, such as infirmities as they age or compression of morbidity, and not the specific life span of an individual.
If age x is subtracted from the hypothetical upper limit w for the species and logs are taken, then the resulting variable log(w – x) is normally distributed similarly as all natural quantitative variables resulting from gene expression. This is due to the law of large numbers, the Central Limit Theorem.
In humans
The longest living person whose dates of birth and death were verified according to the modern norms of Guinness World Records and the Gerontology Research Group was Jeanne Calment (1875–1997), a French woman who reportedly lived to 122. Reduction of infant mortality has accounted for most of the increased average life span longevity, but since the 1960s mortality rates among those over 80 years have decreased by about 1.5% per year. "The progress being made in lengthening lifespans and postponing senescence is entirely due to medical and public-health efforts, rising standards of living, better education, healthier nutrition and more salubrious lifestyles."[3] Animal studies suggest that further lengthening of median human lifespan as well as maximum lifespan could be achieved through "calorie restriction mimetic" drugs or by directly reducing food consumption.[4] Although calorie restriction has not been proven to extend the maximum human life span, as of 2014, results in ongoing primate studies have demonstrated that the assumptions derived from rodents are valid in primates as well [Reference: Nature 1 April 2014].[5]
It has been proposed that no fixed theoretical limit to human longevity is apparent today.[6][7] Studies in the biodemography of human longevity indicate a late-life mortality deceleration law: that death rates level off at advanced ages to a late-life mortality plateau. That is, there is no fixed upper limit to human longevity, or fixed maximal human lifespan.[8] This law was first quantified in 1939, when researchers found that the one-year probability of death at advanced age asymptotically approaches a limit of 44% for women and 54% for men.[9]
However, this evidence depends on the existence of a late-life plateaus and deceleration that can be explained, in humans and other species, by the existence of very rare errors.[10][11] Age-coding error rates below 1 in 10,000 are sufficient to make artificial late-life plateaus, and errors below 1 in 100,000 can generate late-life mortality deceleration. These error rates cannot be ruled out by examining documents,[11] the standard because successful pension fraud, identity theft, forgeries and errors that leave no documentary evidence. This capacity for errors to explain late-life plateaus solves the "fundamental question in aging research is whether humans and other species possess an immutable life-span limit.",[12] indicating that humans do indeed have a lifespan limit.
Scientists have observed that a person's VO2max value (a measure of the volume of oxygen flow to the cardiac muscle) decreases as a function of age. Therefore, the maximum lifespan of a person could be determined by calculating when the person's VO2max value drops below the basal metabolic rate necessary to sustain life, which is approximately 3 ml per kg per minute.[13] On the basis of this hypothesis, athletes with a VO2max value between 50 and 60 at age 20 would be expected "to live for 100 to 125 years, provided they maintained their physical activity so that their rate of decline in VO2max remained constant".[14]
Longitudinal variations of physiological indices, such as complete blood counts (CBC), along individual aging trajectories revealed a linear increase of the organism state fluctuations range with age. The broadening could be explained by a progressive loss of physiological resilience measured by the individual blood factors inverse auto-correlation times. Extrapolation of this data suggested that organism state recovery time and variance would simultaneously diverge at a critical point of 120 – 150 years of age corresponding to a complete loss of resilience and hence should be incompatible with survival. The criticality resulting in the end of life is an intrinsic biological property of an organism that is independent of stress factors and signifies a fundamental or absolute limit of human lifespan.[15]
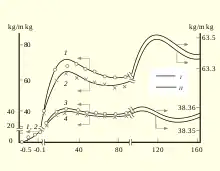
A theoretical study suggested the maximum human lifespan to be around 125 years using a modified stretched exponential function for human survival curves.[17] The analysis of dynamics of the body mass in human population indicates extremums, which correspond to mean (70–75 years), the commonly accepted maximum (100–110 years) and maximum known (140–160 years) lifespan . In another study, researchers claimed that there exists a maximum lifespan for humans, and that the human maximal lifespan has been declining since the 1990s.[18] However, a theoretical study also suggested that the maximum human life expectancy at birth is limited by the human life characteristic value δ, which is around 104 years.[19]
The United Nations has undertaken an important Bayesian sensitivity analysis of global population burden based on life expectancy projection at birth in future decades. The 2017 95% prediction interval of 2090 average life expectancy rises as high as +6 (106, in Century Representation Form) by 2090, with dramatic, ongoing, layered consequences on world population and demography should that happen. The prediction interval is extremely wide, and the United Nations can not be certain. Organizations like the Methuselah Foundation are working toward an end to senescence and practically unlimited human lifespan. If successful, the demographic implications for human population will be greater in effective multiplier terms than any experienced in the last five centuries if maximum lifespan or the birthrate remain unlimited by law. Modern Malthusian predictions of overpopulation based on increased longevity have been criticized on the same basis as general population alarmism (see Malthusianism).
In other animals
Small animals such as birds and squirrels rarely live to their maximum life span, usually dying of accidents, disease or predation.
The maximum life span of most species is documented in the Anage repository.[20]
Maximum life span is usually longer for species that are larger or have effective defenses against predation, such as bird flight,[21] chemical defenses[22] or living in social groups.[23]
The differences in life span between species demonstrate the role of genetics in determining maximum life span ("rate of aging"). The records (in years) are these:
- for common house mouse, 4[24]
- for Brown rat, 3.8[25]
- for dogs, 29 (See List of oldest dogs)[26]
- for cats, 38 (See List of oldest cats)[27]
- for polar bears, 42[28] (Debby)
- for horses, 62[29]
- for Asian elephants, 86[30]
The longest-lived vertebrates have been variously described as
- Large parrots (macaws and cockatoos can live up to 80–100 years in captivity)
- Koi (a Japanese species of fish, allegedly living up to 200 years, though generally not exceeding 50 – a specimen named Hanako was reportedly 226 years old upon her death)[31][32]
- Tortoises (Galápagos tortoise) (190 years)[33]
- Tuataras (a New Zealand reptile species, 100–200+ years[34])
- Eels, the so-called Brantevik Eel (Swedish: Branteviksålen) is thought to have lived in a water well in southern Sweden since 1859, which makes it over 150 years old.[35] It was reported that it had died in August 2014 at an age of 155.[36]
- Whales (bowhead whale) (Balaena mysticetus about 200 years) Although this idea was unproven for a time, recent research has indicated that bowhead whales recently killed still had harpoons in their bodies from about 1890,[37] which, along with analysis of amino acids, has indicated a maximum life span, stated as "the 211 year-old bowhead could have been from 177 to 245 years old".[38][39][40]
- Greenland sharks are currently the vertebrate species with the longest known lifespan.[41] An examination of 28 specimens in one study published in 2016 determined by radiocarbon dating that the oldest of the animals that they sampled had lived for about 392 ± 120 years (a minimum of 272 years and a maximum of 512 years). The authors further concluded that the species reaches sexual maturity at about 150 years of age.[41]
Invertebrate species which continue to grow as long as they live (e.g., certain clams, some coral species) can on occasion live hundreds of years:
- A bivalve mollusk (Arctica islandica) (aka "Ming", lived 507±2 years.[42][43])
Exceptions
- Some jellyfish species, including Turritopsis dohrnii, Laodicea undulata,[44] and Aurelia sp.1,[45] are able to revert to the polyp stage even after reproducing (so-called reversible life cycle), rather than dying as in other jellyfish. Consequently, these species are considered biologically immortal and have no maximum lifespan.[46]
- There may be no natural limit to the Hydra's life span, but it is not yet clear how to estimate the age of a specimen.
- Flatworms, or Platyhelminthes, are known to be "almost immortal" as they have a great regeneration capacity, continuous growth and binary fission type cellular division.[47]
- Lobsters are sometimes said to be biologically immortal because they don't seem to slow down, weaken, or lose fertility with age. However, due to the energy needed for moulting, they cannot live indefinitely.[48]
- Tardigrades can live indefinitely in a state of suspended animation, a state which they enter when they are not hydrated. In this state, they can withstand an extremely large number of environmental pressures, including intense radioactivity and heat, and being sent into space. Despite this, they can only live in a hydrated state for a few months.[49]
In plants
Plants are referred to as annuals which live only one year, biennials which live two years, and perennials which live longer than that. The longest-lived perennials, woody-stemmed plants such as trees and bushes, often live for hundreds and even thousands of years (one may question whether or not they may die of old age). A giant sequoia, General Sherman is alive and well in its third millennium. A Great Basin Bristlecone Pine called Methuselah is 4,848 years old (as of 2017) and the Bristlecone Pine called Prometheus was a little older still, at least 4,844 years (and possibly as old as 5,000 years), when it was cut down in 1964. The oldest known plant (possibly oldest living thing) is a clonal Quaking Aspen (Populus tremuloides) tree colony in the Fishlake National Forest in Utah called Pando at about 80,000 years. Lichen, a symbiotic algae and fungal proto-plant, such as Rhizocarpon geographicum can live upwards of 10,000 years.
Increasing maximum life span
"Maximum life span" here means the mean life span of the most long-lived 10% of a given cohort. Caloric restriction has not yet been shown to break mammalian world records for longevity. Rats, mice, and hamsters experience maximum life-span extension from a diet that contains all of the nutrients but only 40–60% of the calories that the animals consume when they can eat as much as they want. Mean life span is increased 65% and maximum life span is increased 50%, when caloric restriction is begun just before puberty.[50] For fruit flies the life extending benefits of calorie restriction are gained immediately at any age upon beginning calorie restriction and ended immediately at any age upon resuming full feeding.[51]
A few transgenic strains of mice have been created that have maximum life spans greater than that of wild-type or laboratory mice. The Ames and Snell mice, which have mutations in pituitary transcription factors and hence are deficient in Gh, LH, TSH, and secondarily IGF1, have extensions in maximal lifespan of up to 65%. To date, both in absolute and relative terms, these Ames and Snell mice have the maximum lifespan of any mouse not on caloric restriction (see below on GhR). Mutations/knockout of other genes affecting the GH/IGF1 axis, such as Lit, Ghr and Irs1 have also shown extension in lifespan, but much more modest both in relative and absolute terms. The longest lived laboratory mouse ever was a Ghr knockout mouse, which lived to ≈1800 days in the lab of Andrzej Bartke at Southern Illinois University. The maximum for normal B6 mice under ideal conditions is 1200 days.
Most biomedical gerontologists believe that biomedical molecular engineering will eventually extend maximum lifespan and even bring about rejuvenation.Anti-aging drugs are a potential tool for extending life.[52]
Aubrey de Grey, a theoretical gerontologist, has proposed that aging can be reversed by Strategies for Engineered Negligible Senescence. De Grey has established The Methuselah Mouse Prize to award money to researchers who can extend the maximum life span of mice. So far, three Mouse Prizes have been awarded: one for breaking longevity records to Dr. Andrzej Bartke of Southern Illinois University (using GhR knockout mice); one for late-onset rejuvenation strategies to Dr. Stephen Spindler of the University of California (using caloric restriction initiated late in life); and one to Dr. Z. Dave Sharp for his work with the pharmaceutical rapamycin.[53]
Correlation with DNA repair capacity
Accumulated DNA damage appears to be a limiting factor in the determination of maximum life span. The theory that DNA damage is the primary cause of aging, and thus a principal determinant of maximum life span, has attracted increased interest in recent years. This is based, in part, on evidence in human and mouse that inherited deficiencies in DNA repair genes often cause accelerated aging.[54][55][56] There is also substantial evidence that DNA damage accumulates with age in mammalian tissues, such as those of the brain, muscle, liver and kidney (reviewed by Bernstein et al.[57] and see DNA damage theory of aging and DNA damage (naturally occurring)). One expectation of the theory (that DNA damage is the primary cause of aging) is that among species with differing maximum life spans, the capacity to repair DNA damage should correlate with lifespan. The first experimental test of this idea was by Hart and Setlow[58] who measured the capacity of cells from seven different mammalian species to carry out DNA repair. They found that nucleotide excision repair capability increased systematically with species longevity. This correlation was striking and stimulated a series of 11 additional experiments in different laboratories over succeeding years on the relationship of nucleotide excision repair and life span in mammalian species (reviewed by Bernstein and Bernstein[59]). In general, the findings of these studies indicated a good correlation between nucleotide excision repair capacity and life span. The association between nucleotide excision repair capability and longevity is strengthened by the evidence that defects in nucleotide excision repair proteins in humans and rodents cause features of premature aging, as reviewed by Diderich.[55]
Further support for the theory that DNA damage is the primary cause of aging comes from study of Poly ADP ribose polymerases (PARPs). PARPs are enzymes that are activated by DNA strand breaks and play a role in DNA base excision repair. Burkle et al. reviewed evidence that PARPs, and especially PARP-1, are involved in maintaining mammalian longevity.[60] The life span of 13 mammalian species correlated with poly(ADP ribosyl)ation capability measured in mononuclear cells. Furthermore, lymphoblastoid cell lines from peripheral blood lymphocytes of humans over age 100 had a significantly higher poly(ADP-ribosyl)ation capability than control cell lines from younger individuals.
Research data
- A comparison of the heart mitochondria in rats (7-year maximum life span) and pigeons (35-year maximum life span) showed that pigeon mitochondria leak fewer free-radicals than rat mitochondria, despite the fact that both animals have similar metabolic rate and cardiac output[61]
- For mammals there is a direct relationship between mitochondrial membrane fatty acid saturation and maximum life span[62]
- Studies of the liver lipids of mammals and a bird (pigeon) show an inverse relationship between maximum life span and number of double bonds[63]
- Selected species of birds and mammals show an inverse relationship between telomere rate of change (shortening) and maximum life span[64]
- Maximum life span correlates negatively with antioxidant enzyme levels and free-radicals production and positively with rate of DNA repair[65]
- Female mammals express more Mn−SOD and glutathione peroxidase antioxidant enzymes than males. This has been hypothesized as the reason they live longer[66] However, mice entirely lacking in glutathione peroxidase 1 do not show a reduction in lifespan.
- The maximum life span of transgenic mice has been extended about 20% by overexpression of human catalase targeted to mitochondria[67]
- A comparison of 7 non-primate mammals (mouse, hamster, rat, guinea-pig, rabbit, pig and cow) showed that the rate of mitochondrial superoxide and hydrogen peroxide production in heart and kidney were inversely correlated with maximum life span[68]
- A study of 8 non-primate mammals showed an inverse correlation between maximum life span and oxidative damage to mtDNA (mitochondrial DNA) in heart & brain[69]
- A study of several species of mammals and a bird (pigeon) indicated a linear relationship between oxidative damage to protein and maximum life span[70]
- There is a direct correlation between DNA repair and maximum life span for mammalian species[71]
- Drosophila (fruit-flies) bred for 15 generations by only using eggs that were laid toward the end of reproductive life achieved maximum life spans 30% greater than that of controls[72]
- Overexpression of the enzyme which synthesizes glutathione in long-lived transgenic Drosophila (fruit-flies) extended maximum lifespan by nearly 50%[73]
- A mutation in the age−1 gene of the nematode worm Caenorhabditis elegans increased mean life span 65% and maximum life span 110%.[74] However, the degree of lifespan extension in relative terms by both the age-1 and daf-2 mutations is strongly dependent on ambient temperature, with ≈10% extension at 16 °C and 65% extension at 27 °C.
- Fat-specific Insulin Receptor KnockOut (FIRKO) mice have reduced fat mass, normal calorie intake and an increased maximum life span of 18%.[75]
- The capacity of mammalian species to detoxify the carcinogenic chemical benzo(a)pyrene to a water-soluble form also correlates well with maximum life span.[76]
- Short-term induction of oxidative stress due to calorie restriction increases life span in Caenorhabditis elegans by promoting stress defense, specifically by inducing an enzyme called catalase. As shown by Michael Ristow and co-workers nutritive antioxidants completely abolish this extension of life span by inhibiting a process called mitohormesis.[77]
See also
- Ageing
- Aging brain
- American Aging Association
- Aubrey de Grey
- Biodemography
- Biological immortality
- Calorie restriction
- Compression of morbidity
- DNA damage theory of aging
- Extreme longevity tracking
- Genetics of aging
- Gerontology
- Hayflick limit
- Indefinite lifespan
- Life expectancy
- Life extension
- List of long-living organisms
- Longevity
- Methuselah Mouse Prize
- Michael Ristow
- Mitohormesis
- Oldest people
- Senescence
- Strategies for Engineered Negligible Senescence (SENS)
References
- Gavrilov LA, Gavrilova NS (1991). The Biology of Life Span: A Quantitative Approach. New York: Harwood Academic. ISBN 978-3-7186-4983-9.
- Brody JE (25 August 2008). "Living Longer, in Good Health to the End". The New York Times. p. D7.
- Vaupel JW (March 2010). "Biodemography of human ageing". Nature. 464 (7288): 536–42. Bibcode:2010Natur.464..536V. doi:10.1038/nature08984. PMC 4010874. PMID 20336136.
- Ben-Haim MS, Kanfi Y, Mitchell SJ, Maoz N, Vaughan KL, Amariglio N, Lerrer B, de Cabo R, Rechavi G, Cohen HY (October 2018). "Breaking the Ceiling of Human Maximal Life span". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 73 (11): 1465–1471. doi:10.1093/gerona/glx219. PMC 6454488. PMID 29121176.
- Ingram DK, Roth GS, Lane MA, Ottinger MA, Zou S, de Cabo R, Mattison JA (June 2006). "The potential for dietary restriction to increase longevity in humans: extrapolation from monkey studies". Biogerontology. 7 (3): 143–8. doi:10.1007/s10522-006-9013-2. PMID 16732404. S2CID 2859875.
- Gavrilov LA, Gavrilova NS (1991). The Biology of Life Span: A Quantitative Approach. New York City: Starwood Academic Publishers.
- Gavrilov LA, Gavrilova NS (June 2000). "Book Reviews: Validation of Exceptional Longevity" (PDF). Population Dev Rev. 26 (2): 403–04. Retrieved 2009-05-18.
- Gavrilov LA (5 March 2004). "Biodemography of Human Longevity". International Conference on Longevity. Retrieved 2018-01-13.
- Greenwood M, Irwin JO (1939). "The Biostatics of Senility" (PDF). Human Biology. 11: 1–23. Retrieved 2009-05-18.
- Gavrilova NS, Gavrilov LA (2014). "Mortality Trajectories at Extreme Old Ages: A Comparative Study of Different Data Sources on U.S. Old-Age Mortality". Living to 100 Monograph. 2014. PMC 4318539. PMID 25664347.
- Newman SJ (December 2018). "Errors as a primary cause of late-life mortality deceleration and plateaus". PLOS Biology. 16 (12): e2006776. doi:10.1371/journal.pbio.2006776. PMC 6301557. PMID 30571676.
- Wilmoth JR, Deegan LJ, Lundström H, Horiuchi S (September 2000). "Increase of maximum life-span in Sweden, 1861-1999". Science. 289 (5488): 2366–8. Bibcode:2000Sci...289.2366W. doi:10.1126/science.289.5488.2366. PMID 11009426.
- Noakes T (1985). The Lore of Running. Oxford University Press.
- Nokes (1985) p. 84.
- Pyrkov, T.; Avchaciov, K.; Tarkhov, A.; Menshikov, L.; Gudkov, A.; Fedichev, P. (26 April 2019). "Longitudinal analysis of blood markers reveals progressive loss of resilience and predicts ultimate limit of human lifespan". bioRxiv 10.1101/618876.
- Gerasimov IG, Ignatov DY (2004). "Age Dynamics of Body Mass and Human Lifespan". Journal of Evolutionary Biochemistry and Physiology. 40 (3): 343–349. doi:10.1023/B:JOEY.0000042639.72529.e1. S2CID 9070790.
- Weon BM, Je JH (February 2009). "Theoretical estimation of maximum human lifespan". Biogerontology. 10 (1): 65–71. doi:10.1007/s10522-008-9156-4. PMID 18560989. S2CID 8554128.
- Dong X, Milholland B, Vijg J (October 2016). "Evidence for a limit to human lifespan". Nature. 538 (7624): 257–259. Bibcode:2016Natur.538..257D. doi:10.1038/nature19793. PMID 27706136. S2CID 3623127.
- Liu X (December 2015). "Life equations for the senescence process". Biochemistry and Biophysics Reports. 4: 228–233. doi:10.1016/j.bbrep.2015.09.020. PMC 5669524. PMID 29124208.
- "The Animal Ageing and Longevity Database". Anage.
- Healy K, Guillerme T, Finlay S, Kane A, Kelly SB, McClean D, Kelly DJ, Donohue I, Jackson AL, Cooper N (June 2014). "Ecology and mode-of-life explain lifespan variation in birds and mammals". Proceedings. Biological Sciences. 281 (1784): 20140298. doi:10.1098/rspb.2014.0298. PMC 4043093. PMID 24741018.
- Hossie TJ, Hassall C, Knee W, Sherratt TN (July 2013). "Species with a chemical defence, but not chemical offence, live longer". Journal of Evolutionary Biology. 26 (7): 1598–602. doi:10.1111/jeb.12143. PMID 23638626.
- Krause J, Ruxton G (19 December 2002). Living in Groups (1st ed.). Oxford University Press. ISBN 9780198508182.
- "Longevity, Aging, and Life History of Mus musculus". Retrieved 2009-08-13. Cite journal requires
|journal=(help) - "Norway rat (Rattus norvegicus) longevity, ageing, and life history". genomics.senescence.info. Retrieved 2017-03-15.
- "Max misses 'World's Oldest Dog' title". iberianet.com.
- Guinness World Records 2010. Bantam. 2010. p. 320. ISBN 978-0-553-59337-2.
The oldest cat ever was Creme Puff, who was born on August 3, 1967, and lived until August 6, 2005—38 years and 3 days in total.
- "World's oldest polar bear". Archived from the original on 3 August 2009. Retrieved 2008-11-19.
- Ensminger, M. E. (1990). Horses and Horsemanship: Animal Agricultural Series (Sixth ed.). Danville, Indiana: Interstate Publishers. ISBN 978-0-8134-2883-3. OCLC 21977751., pp. 46–50
- "Lin Wang, an Asian elephant (Elephas maximus) at Taipei Zoo". Retrieved 2009-08-13.
- "International Nishikigoi Promotion Center-Genealogy". Japan-nishikigoi.org. Retrieved 2009-04-11.
- Barton L (12 April 2007). "Will you still feed me ... ?". The Guardian. London. Retrieved 2009-04-11.
- Seed: Week In Science: 6/23 - 6/29 Archived 31 October 2007 at the Wayback Machine
- Tuatara#cite note-43
- "Brantevik Eels may be the world's oldest". 11 April 2008. Archived from the original on 13 August 2010.
- "The world's oldest Eek dead - Lived 155 years in a well (Article in Swedish )". 8 August 2014.
- "125-Year-old New Bedford Bomb Fragment Found Embedded in Alaskan Bowhead Whale". Archived from the original on 28 July 2011.
- "Bowhead Whales May Be the World's Oldest Mammals". 2001. Archived from the original on 2009-12-09. Retrieved 2019-01-05.
- "Bowhead Whales May Be the World's Oldest Mammals". 2007 [2001].
- George JC, Bada J, Zeh J, Scott L, Brown SE, O'hara T, Suydam R (1999). "Age and growth estimates of bowhead whales (Balaena mysticetus) via aspartic acid racemization". Canadian Journal of Zoology. 77 (4): 571–580. doi:10.1139/cjz-77-4-571.
- Nielsen J, Hedeholm RB, Heinemeier J, Bushnell PG, Christiansen JS, Olsen J, Ramsey CB, Brill RW, Simon M, Steffensen KF, Steffensen JF (August 2016). "Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus)". Science. 353 (6300): 702–4. Bibcode:2016Sci...353..702N. doi:10.1126/science.aaf1703. PMID 27516602. S2CID 206647043. Lay summary – Science News (12 August 2016).
- Butler PG, Wanamaker AD, Scourse JD, Richardson CA, Reynolds DJ (March 2013). "Variability of marine climate on the North Icelandic Shelf in a 1357-year proxy archive based on growth increments in the bivalve Arctica islandica". Palaeogeography, Palaeoclimatology, Palaeoecology. 373: 141–51. Bibcode:2013PPP...373..141B. doi:10.1016/j.palaeo.2012.01.016.
- Brix L (6 November 2013). "New record: World's oldest animal is 507 years old". Sciencenordic. Archived from the original on 15 November 2013. Retrieved 2013-11-14.
- De Vito D, Piraino S, Schmich J, Bouillon J, Boero F (2006). "Evidence of reverse development in Leptomedusae (Cnidaria, Hydrozoa): the case of Laodicea undulata (Forbes and Goodsir 1851) o". Marine Biology. 149 (2): 339–346. doi:10.1007/s00227-005-0182-3. S2CID 84325535.
- He J, Zheng L, Zhang W, Lin Y (21 December 2015). "Life Cycle Reversal in Aurelia sp.1 (Cnidaria, Scyphozoa)". PLOS ONE. 10 (12): e0145314. Bibcode:2015PLoSO..1045314H. doi:10.1371/journal.pone.0145314. PMC 4687044. PMID 26690755.
- Piraino S, Boero F, Aeschbach B, Schmid V (June 1996). "Reversing the Life Cycle: Medusae Transforming into Polyps and Cell Transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa)". The Biological Bulletin. 190 (3): 302–312. doi:10.2307/1543022. JSTOR 1543022. PMID 29227703.
- Saló E (May 2006). "The power of regeneration and the stem-cell kingdom: freshwater planarians (Platyhelminthes)". BioEssays. 28 (5): 546–59. doi:10.1002/bies.20416. PMID 16615086.
- Marina Koren (3 June 2013). "Don't Listen to the Buzz: Lobsters Aren't Actually Immortal". Smithsonian.com.
- "The Secret of the Only Animal That Can Survive in Space". 2014-08-07.
- Koubova J, Guarente L (February 2003). "How does calorie restriction work?". Genes & Development. 17 (3): 313–21. doi:10.1101/gad.1052903. PMID 12569120.
- Mair W, Goymer P, Pletcher SD, Partridge L (September 2003). "Demography of dietary restriction and death in Drosophila". Science. 301 (5640): 1731–3. Bibcode:2003Sci...301.1731M. doi:10.1126/science.1086016. PMID 14500985. S2CID 27653353.
- Kaeberlein M (February 2010). "Resveratrol and rapamycin: are they anti-aging drugs?". BioEssays. 32 (2): 96–9. doi:10.1002/bies.200900171. PMID 20091754.
- "Work". Methuselah Foundation. Archived from the original on 2015-02-14. Retrieved 2018-12-10.
- Hoeijmakers JH (October 2009). "DNA damage, aging, and cancer". The New England Journal of Medicine. 361 (15): 1475–85. doi:10.1056/NEJMra0804615. PMID 19812404.
- Diderich K, Alanazi M, Hoeijmakers JH (July 2011). "Premature aging and cancer in nucleotide excision repair-disorders". DNA Repair. 10 (7): 772–80. doi:10.1016/j.dnarep.2011.04.025. PMC 4128095. PMID 21680258.
- Freitas AA, de Magalhães JP (2011). "A review and appraisal of the DNA damage theory of ageing". Mutation Research. 728 (1–2): 12–22. doi:10.1016/j.mrrev.2011.05.001. PMID 21600302.
- Bernstein H, Payne CM, Bernstein C, Garewal H, Dvorak K (2008). "Chapter 1: Cancer and aging as consequences of un-repaired DNA damage.". In Kimura H, Suzuki A (eds.). New Research on DNA Damages. New York: Nova Science Publishers, Inc. pp. 1–47. ISBN 978-1-60456-581-2.
- Hart RW, Setlow RB (June 1974). "Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species". Proceedings of the National Academy of Sciences of the United States of America. 71 (6): 2169–73. Bibcode:1974PNAS...71.2169H. doi:10.1073/pnas.71.6.2169. PMC 388412. PMID 4526202.
- Bernstein C, Bernstein H (1991). Aging, Sex, and DNA Repair. San Diego: Academic Press. ISBN 978-0-12-092860-6.
- Bürkle A, Brabeck C, Diefenbach J, Beneke S (May 2005). "The emerging role of poly(ADP-ribose) polymerase-1 in longevity". The International Journal of Biochemistry & Cell Biology. 37 (5): 1043–53. doi:10.1016/j.biocel.2004.10.006. PMID 15743677.
- Herrero A, Barja G (November 1997). "Sites and mechanisms responsible for the low rate of free radical production of heart mitochondria in the long-lived pigeon". Mechanisms of Ageing and Development. 98 (2): 95–111. doi:10.1016/S0047-6374(97)00076-6. PMID 9379714. S2CID 20424838.
- Pamplona R, Portero-Otín M, Riba D, Ruiz C, Prat J, Bellmunt MJ, Barja G (October 1998). "Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals". Journal of Lipid Research. 39 (10): 1989–94. PMID 9788245.
- Pamplona R, Portero-Otín M, Riba D, Requena JR, Thorpe SR, López-Torres M, Barja G (June 2000). "Low fatty acid unsaturation: a mechanism for lowered lipoperoxidative modification of tissue proteins in mammalian species with long life spans". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 55 (6): B286–91. doi:10.1093/gerona/55.6.b286. PMID 10843345.
- Haussmann MF, Winkler DW, O'Reilly KM, Huntington CE, Nisbet IC, Vleck CM (July 2003). "Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones". Proceedings. Biological Sciences. 270 (1522): 1387–92. doi:10.1098/rspb.2003.2385. PMC 1691385. PMID 12965030.
- Perez-Campo R, López-Torres M, Cadenas S, Rojas C, Barja G (April 1998). "The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach". Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology. 168 (3): 149–58. doi:10.1007/s003600050131. PMID 9591361. S2CID 12080649.
- Viña J, Borrás C, Gambini J, Sastre J, Pallardó FV (May 2005). "Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds". FEBS Letters. 579 (12): 2541–5. doi:10.1016/j.febslet.2005.03.090. PMID 15862287.
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS (June 2005). "Extension of murine life span by overexpression of catalase targeted to mitochondria". Science. 308 (5730): 1909–11. Bibcode:2005Sci...308.1909S. doi:10.1126/science.1106653. PMID 15879174. S2CID 38568666.
- Ku HH, Brunk UT, Sohal RS (December 1993). "Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species". Free Radical Biology & Medicine. 15 (6): 621–7. doi:10.1016/0891-5849(93)90165-Q. PMID 8138188.
- Barja G, Herrero A (February 2000). "Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals". FASEB Journal. 14 (2): 312–8. doi:10.1096/fasebj.14.2.312. PMID 10657987.
- Agarwal S, Sohal RS (1996). "Relationship between susceptibility to protein oxidation, aging, and maximum life span potential of different species". Experimental Gerontology. 31 (3): 365–72. doi:10.1016/0531-5565(95)02039-X. PMID 9415119. S2CID 21564827.
- Cortopassi GA, Wang E (November 1996). "There is substantial agreement among interspecies estimates of DNA repair activity". Mechanisms of Ageing and Development. 91 (3): 211–8. doi:10.1016/S0047-6374(96)01788-5. PMID 9055244. S2CID 24364141.
- Kurapati R, Passananti HB, Rose MR, Tower J (November 2000). "Increased hsp22 RNA levels in Drosophila lines genetically selected for increased longevity". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 55 (11): B552–9. doi:10.1093/gerona/55.11.b552. PMID 11078089.
- Orr WC, Radyuk SN, Prabhudesai L, Toroser D, Benes JJ, Luchak JM, Mockett RJ, Rebrin I, Hubbard JG, Sohal RS (November 2005). "Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster". The Journal of Biological Chemistry. 280 (45): 37331–8. doi:10.1074/jbc.M508272200. PMID 16148000.
- Friedman DB, Johnson TE (January 1988). "A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility". Genetics. 118 (1): 75–86. PMC 1203268. PMID 8608934.
- Blüher M, Kahn BB, Kahn CR (January 2003). "Extended longevity in mice lacking the insulin receptor in adipose tissue". Science. 299 (5606): 572–4. Bibcode:2003Sci...299..572B. doi:10.1126/science.1078223. PMID 12543978. S2CID 24114184.
- Moore CJ, Schwartz AG (October 1978). "Inverse correlation between species lifespan and capacity of cultured fibroblasts to convert benzo(a)pyrene to water-soluble metabolites". Experimental Cell Research. 116 (2): 359–64. doi:10.1016/0014-4827(78)90459-7. PMID 101383.
- "Publication demonstrating that oxidative stress is promoting life span". Cellmetabolism.org. Archived from the original on 2012-07-03. Retrieved 2010-11-04. Cite journal requires
|journal=(help)