Ageing
Ageing or aging (see spelling differences) is the process of becoming older. The term refers especially to humans, many other animals, and fungi, whereas for example bacteria, perennial plants and some simple animals are potentially biologically immortal. In the broader sense, ageing can refer to single cells within an organism which have ceased dividing (cellular senescence) or to the population of a species (population ageing).
| Part of a series on |
| Human growth and development |
|---|
 |
| Stages |
| Biological milestones |
| Development and psychology |
|
In humans, ageing represents the accumulation of changes in a human being over time[1] and can encompass physical, psychological, and social changes. Reaction time, for example, may slow with age, while knowledge of world events and wisdom may expand. Ageing is among the greatest known risk factors for most human diseases: of the roughly 150,000 people who die each day across the globe, about two-thirds die from age-related causes.
The causes of ageing are uncertain; current theories are assigned to the damage concept, whereby the accumulation of damage (such as DNA oxidation) may cause biological systems to fail, or to the programmed ageing concept, whereby problems with the internal processes (epigenomic maintenance such as DNA methylation [2]) may cause ageing. Programmed ageing should not be confused with programmed cell death (apoptosis). Additionally, there can be other reasons, which can speed up the rate of ageing in organisms including human beings like obesity [3][4] and compromised immune system.
Definitions
Mortality can be used to define biological ageing, which refers to an organism's increased rate of death as it progresses throughout its lifecycle and increases its chronological age.[5] Another possible way to define ageing is through functional definitions, of which there are two main types[5] The first describes how varying types of deteriorative changes that accumulate in the life of a post-maturation organism can leave it vulnerable, leading to a decreased ability of the organism to survive. The second is a senescence-based definition; this describes age-related changes in an organism that increase its mortality rate over time by negatively affecting its vitality and functional performance.[5] An important distinction to make is that biological ageing is not the same thing as the accumulation of diseases related to old age; disease is a blanket term used to describe a process within an organism that causes a decrease in its functional ability.[5]
Ageing versus immortality
.JPG.webp)
Human beings and members of other species, especially animals, age and die. Fungi, too, can age.[6] In contrast, many species can be considered immortal: for example, bacteria fission to produce daughter cells, strawberry plants grow runners to produce clones of themselves, and animals in the genus Hydra have a regenerative ability by which they avoid dying of old age.
Early life forms on Earth, starting at least 3.7 billion years ago,[7] were single-celled organisms. Such organisms (Prokaryotes, Protozoans, algae) multiply by fission into daughter cells; thus do not age and are innately immortal.[8][9]
Ageing and mortality of the individual organism became possible with the evolution of sexual reproduction,[10] which occurred with the emergence of the fungal/animal kingdoms approximately a billion years ago, and the evolution of seed-producing plants 320 million years ago. The sexual organism could henceforth pass on some of its genetic material to produce new individuals and could itself become disposable with respect to the survival of its species.[10] This classic biological idea has however been perturbed recently by the discovery that the bacterium E. coli may split into distinguishable daughter cells, which opens the theoretical possibility of "age classes" among bacteria.[11]
Even within humans and other mortal species, there are cells with the potential for immortality: cancer cells which have lost the ability to die when maintained in a cell culture such as the HeLa cell line,[12] and specific stem cells such as germ cells (producing ova and spermatozoa).[13] In artificial cloning, adult cells can be rejuvenated to embryonic status and then used to grow a new tissue or animal without ageing.[14] Normal human cells however die after about 50 cell divisions in laboratory culture (the Hayflick Limit, discovered by Leonard Hayflick in 1961).[12]
Effects

A number of characteristic ageing symptoms are experienced by a majority or by a significant proportion of humans during their lifetimes.
- Teenagers lose the young child's ability to hear high-frequency sounds above 20 kHz.[17]
- Wrinkles develop mainly due to photoageing, particularly affecting sun-exposed areas (face).[18]
- After peaking in the mid-20s, female fertility declines.[19]
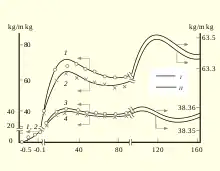
- After age 30 the mass of human body is decreased until 70 years and then shows damping oscillations.[16]
- Muscles have reduced capacity of responding to exercise or injury and loss of muscle mass and strength (sarcopenia) is common.[20] VO2 max and maximum heart rate decline.[21]
- Hand strength and mobility are decreased during the aging process. These things include, "hand and finger strength and ability to control submaximal pinch force and maintain a steady precision pinch posture, manual speed, and hand sensation"[22]
- People over 35 years of age are at increasing risk for losing strength in the ciliary muscle which leads to difficulty focusing on close objects, or presbyopia.[23][24] Most people experience presbyopia by age 45–50.[25] The cause is lens hardening by decreasing levels of α-crystallin, a process which may be sped up by higher temperatures.[25][26]
- Around age 50, hair turns grey.[27] Pattern hair loss by the age of 50 affects about 30–50% of males[28] and a quarter of females.[29]
- Menopause typically occurs between 44 and 58 years of age.[30]
- In the 60–64 age cohort, the incidence of osteoarthritis rises to 53%. Only 20% however report disabling osteoarthritis at this age.[31]
- Almost half of people older than 75 have hearing loss (presbycusis) inhibiting spoken communication.[32] Many vertebrates such as fish, birds and amphibians do not suffer presbycusis in old age as they are able to regenerate their cochlear sensory cells, whereas mammals including humans have genetically lost this ability.[33]
- By age 80, more than half of all Americans either have a cataract or have had cataract surgery.[34]
- Frailty, a syndrome of decreased strength, physical activity, physical performance and energy, affects 25% of those over 85.[35][36]
- Atherosclerosis is classified as an ageing disease.[37] It leads to cardiovascular disease (for example stroke and heart attack)[38] which globally is the most common cause of death.[39] Vessel ageing causes vascular remodeling and loss of arterial elasticity and as a result causes the stiffness of the vasculature.[37]
- Recent evidence suggests that age-related risk of death plateaus after age 105.[40] The maximum human lifespan is suggested to be 115 years.[41][42] The oldest reliably recorded human was Jeanne Calment who died in 1997 at 122.
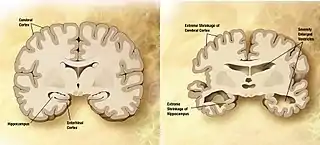
Dementia becomes more common with age.[43] About 3% of people between the ages of 65 and 74, 19% between 75 and 84, and nearly half of those over 85 years of age have dementia.[44] The spectrum ranges from mild cognitive impairment to the neurodegenerative diseases of Alzheimer's disease, cerebrovascular disease, Parkinson's disease and Lou Gehrig's disease. Furthermore, many types of memory decline with ageing, but not semantic memory or general knowledge such as vocabulary definitions, which typically increases or remains steady until late adulthood[45] (see Ageing brain). Intelligence declines with age, though the rate varies depending on the type and may in fact remain steady throughout most of the lifespan, dropping suddenly only as people near the end of their lives. Individual variations in rate of cognitive decline may therefore be explained in terms of people having different lengths of life.[46] There are changes to the brain: after 20 years of age there is a 10% reduction each decade in the total length of the brain's myelinated axons.[47][48]
Age can result in visual impairment, whereby non-verbal communication is reduced,[49] which can lead to isolation and possible depression. Older adults, however, may not suffer depression as much as younger adults, and were paradoxically found to have improved mood despite declining physical health.[50] Macular degeneration causes vision loss and increases with age, affecting nearly 12% of those above the age of 80.[51] This degeneration is caused by systemic changes in the circulation of waste products and by growth of abnormal vessels around the retina.[52]
A distinction can be made between "proximal ageing" (age-based effects that come about because of factors in the recent past) and "distal ageing" (age-based differences that can be traced to a cause in a person's early life, such as childhood poliomyelitis).[46]
Ageing is among the greatest known risk factors for most human diseases.[53] Of the roughly 150,000 people who die each day across the globe, about two-thirds—100,000 per day—die from age-related causes. In industrialized nations, the proportion is higher, reaching 90%.[54][55][56]
Biological basis

At present, researchers are only just beginning to understand the biological basis of ageing even in relatively simple and short-lived organisms such as yeast.[57] Less still is known of mammalian ageing, in part due to the much longer lives of even small mammals such as the mouse (around 3 years). A model organism for studying of ageing is the nematode C. elegans. Thanks to its short lifespan of 2–3 weeks, our ability to easily perform genetic manipulations or to suppress gene activity with RNA interference, or other factors.[58] Most known mutations and RNA interference targets that extend lifespan were first discovered in C. elegans.[59]
The factors proposed to influence biological ageing[60] fall into two main categories, programmed and damage-related. Programmed factors follow a biological timetable, perhaps one that might be a continuation of the one that regulates childhood growth and development. This regulation would depend on changes in gene expression that affect the systems responsible for maintenance, repair and defense responses. Damage-related factors include internal and environmental assaults to living organisms that induce cumulative damage at various levels.[61] A third, novel, concept is that ageing is mediated by vicious cycles.[53]
In a detailed review, Lopez-Otin and colleagues (2013), who discuss ageing through the lens of the damage theory, propose nine metabolic "hallmarks" of ageing in various organisms but especially mammals:[62]
- genomic instability (mutations accumulated in nuclear DNA, in mtDNA, and in the nuclear lamina)
- telomere attrition (the authors note that artificial telomerase confers non-cancerous immortality to otherwise mortal cells)
- epigenetic alterations (including DNA methylation patterns, post-translational modification of histones, and chromatin remodelling)
- loss of proteostasis (protein folding and proteolysis)
- deregulated nutrient sensing (relating to the Growth hormone/Insulin-like growth factor 1 signalling pathway, which is the most conserved ageing-controlling pathway in evolution and among its targets are the FOXO3/Sirtuin transcription factors and the mTOR complexes, probably responsive to caloric restriction)
- mitochondrial dysfunction (the authors point out however that a causal link between ageing and increased mitochondrial production of reactive oxygen species is no longer supported by recent research)
- cellular senescence (accumulation of no longer dividing cells in certain tissues, a process induced especially by p16INK4a/Rb and p19ARF/p53 to stop cancerous cells from proliferating)
- stem cell exhaustion (in the authors' view caused by damage factors such as those listed above)
- altered intercellular communication (encompassing especially inflammation but possibly also other intercellular interactions)
There are three main metabolic pathways which can influence the rate of ageing, discussed below:
- the FOXO3/Sirtuin pathway, probably responsive to caloric restriction
- the Growth hormone/Insulin-like growth factor 1 signalling pathway
- the activity levels of the electron transport chain in mitochondria[63] and (in plants) in chloroplasts.
It is likely that most of these pathways affect ageing separately, because targeting them simultaneously leads to additive increases in lifespan.[64]
Programmed factors
The rate of ageing varies substantially across different species, and this, to a large extent, is genetically based. For example, numerous perennial plants ranging from strawberries and potatoes to willow trees typically produce clones of themselves by vegetative reproduction and are thus potentially immortal, while annual plants such as wheat and watermelons die each year and reproduce by sexual reproduction. In 2008 it was discovered that inactivation of only two genes in the annual plant Arabidopsis thaliana leads to its conversion into a potentially immortal perennial plant.[65] The oldest animals known so far are 15,000-year-old Antarctic sponges,[66] which can reproduce both sexually and clonally.
Clonal immortality apart, there are certain species whose individual lifespans stand out among Earth's life-forms, including the bristlecone pine at 5062 years[67] or 5067 years,[66] invertebrates like the hard clam (known as quahog in New England) at 508 years,[68] the Greenland shark at 400 years,[69] various deep-sea tube worms at over 300 years,[70] fish like the sturgeon and the rockfish, and the sea anemone[71] and lobster.[72][73] Such organisms are sometimes said to exhibit negligible senescence.[74] The genetic aspect has also been demonstrated in studies of human centenarians.
In laboratory settings, researchers have demonstrated that selected alterations in specific genes can extend lifespan quite substantially in yeast and roundworms, less so in fruit flies and less again in mice. Some of the targeted genes have homologues across species and in some cases have been associated with human longevity.[75] Studies by Becca Levy, an associate professor of epidemiology and psychology at the Yale School of Public Health, have found that positive beliefs about ageing may also increase life span.[76]
- DNA methylation: The strong effect of age on DNA methylation levels has been known since the late 1960s.[77] Horvath hypothesised that DNA methylation age measures the cumulative effect of an epigenetic maintenance system but details are unknown. DNA methylation age of blood predicts all-cause mortality in later life.[78][79][80] Furthermore, prematurely aged mice can be rejuvenated and their lives extended by 30% by partially "resetting" the methylation pattern in their cells (a full reset leads to undesirable immortal cancer cells). This resetting into a juvenile state was experimentally achieved in 2016 by activating the four Yamanaka DNA transcription factors – Sox2, Oct4, Klf4 and c-Myc (which have previously been routinely used for producing young animals from cloned adult skin cells).[81][82]
- Senescent cells: most of the cells with DNA damages that can't be fixed do apoptosis but some cells don't. Those cells are related to many diseases such as kidney failure and diabetes. In 2016, in a study, the removal of those cells in mice has extended their lifespans by 20% to 30%.[83] Another study shows that this issue is related to the p16INK4a and β-galactosidase.[84] Significant results have been obtained by some companies to extend mouse lifespan focusing on senescent cells.[85]
- A variation in the gene FOXO3A has a positive effect on the life expectancy of humans, and is found much more often in people living to 100 and beyond – moreover, this appears to be true worldwide.[86][87] FOXO3A acts on the sirtuin family of genes which also have a significant effect on lifespan in yeast and in nematodes. Sirtuin in turn inhibits mTOR.[88]
- Caloric restriction leads to longer lifespans in various species, an effect that is unclear,[64] but probably mediated by the nutrient-sensing function of the mTOR pathway.[89]
- mTOR, a protein that inhibits autophagy, has been linked to ageing through the insulin signalling pathway. mTOR functions through nutrient and growth cues leading scientists to believe that dietary restriction and mTOR are related in terms of longevity. When organisms restrict their diet, mTOR activity is reduced, which allows an increased level of autophagy. This recycles old or damaged cell parts, which increases longevity and decreases the chances of being obese. This is thought to prevent spikes of glucose concentration in the blood, leading to reduced insulin signalling. This has been linked to less mTOR activation as well. Therefore, longevity has been connected to caloric restriction and insulin sensitivity inhibiting mTOR, which in turns allows autophagy to occur more frequently. It may be that mTOR inhibition and autophagy reduce the effects of reactive oxygen species on the body, which damage DNA and other organic material, so longevity would be increased.[90] In support of this contention are observations that several purported anti-ageing remedies such as rapamycin, metformin, berberine, 2-deoxyglucose, vitamin D3, aspirin and resveratrol were shown to suppress mTOR signaling and concurrently to reduce constitutive level of oxidative DNA damage induced by endogenous oxidants as well as to enhance the rate of autophagy[91]
- A decreased Growth hormone/Insulin-like Growth Factor 1 signalling pathway has been associated with increased life span in various organisms including fruit flies, nematodes and mice. The precise mechanism by which decreased GH/IGF-1 signalling increases longevity is unknown, but various mouse strains with decreased GH and/or IGF-1 induced signalling share a similar phenotype which includes increased insulin sensitivity, enhanced stress resistance and protection from carcinogenesis. The studied mouse strains with decreased GH signalling showed between 20% and 68% increased longevity, and mouse strains with decreased IGF-1 induced signalling revealed a 19 to 33% increase in life span when compared to control mice.[92]
- Over-expression of the Ras2 gene increases lifespan in yeast by 30%.[93] A yeast mutant lacking the genes SCH9 and RAS1 has recently been shown to have a tenfold increase in lifespan under conditions of calorie restriction and is the largest increase achieved in any organism.[94][95]
- Telomeres: In humans and other animals, cellular senescence has been attributed to the shortening of telomeres at each cell division;[96] when telomeres become too short, the cells senesce and die or cease multiplying.[97] The length of telomeres is therefore the "molecular clock", predicted by Hayflick.[98][99] However, telomere length in wild mouse strains is unrelated to lifespan,[100] and mice lacking the enzyme telomerase do not have a dramatically reduced lifespan.[101] Laboratory mice's telomeres are many times longer than human ones.[102] Another caveat is that a study following nearly 1000 humans for ten years showed that while some humans do shorten their telomeres over time, a third of the participants did not.[103]
- Evolution of ageing: Many have argued that life span, like other phenotypes, is selected. Traits that benefit early survival and reproduction will be selected for even if they contribute to an earlier death. Such a genetic effect is called the antagonistic pleiotropy effect when referring to a gene (pleiotropy signifying the gene has a double function – enabling reproduction at a young age but costing the organism life expectancy in old age) and is called the disposable soma effect when referring to an entire genetic programme (the organism diverting limited resources from maintenance to reproduction).[10] The biological mechanisms which regulate lifespan evolved several hundred million years ago.[59]
- Some evidence is provided by oxygen-deprived bacterial cultures.[104]
- The theory would explain why the autosomal dominant disease, Huntington's disease, can persist even though it is inexorably lethal. Also, it has been suggested that some of the genetic variants that increase fertility in the young increase cancer risk in the old. Such variants occur in genes p53[105] and BRCA1.[106]
- The reproductive-cell cycle theory argues that ageing is regulated specifically by reproductive hormones that act in an antagonistic pleiotropic manner via cell cycle signalling, promoting growth and development early in life to achieve reproduction, but becoming dysregulated later in life, driving senescence (dyosis) in a futile attempt to maintain reproductive ability.[1][107] The endocrine dyscrasia that follows the loss of follicles with menopause, and the loss of Leydig and Sertoli cells during andropause, drive aberrant cell cycle signalling that leads to cell death and dysfunction, tissue dysfunction (disease) and ultimately death. Moreover, the hormones that regulate reproduction also regulate cellular metabolism, explaining the increases in fat deposition during pregnancy through to the deposition of centralised adiposity with the dysregulation of the HPG axis following menopause and during andropause (Atwood and Bowen, 2004). This theory, which introduced a new definition of ageing, has facilitated the conceptualisation of why and how ageing occurs at the evolutionary, physiological and molecular levels.[1]
- Autoimmunity: The idea that ageing results from an increase in autoantibodies that attack the body's tissues. A number of diseases associated with ageing, such as atrophic gastritis and Hashimoto's thyroiditis, are probably autoimmune in this way. However, while inflammation is very much evident in old mammals, even completely immunodeficient mice raised in pathogen-free laboratory conditions still experience senescence.

- The cellular balance between energy generation and consumption (energy homeostasis) requires tight regulation during ageing. In 2011, it was demonstrated that acetylation levels of AMP-activated protein kinase change with age in yeast and that preventing this change slows yeast ageing.[108]
- Skin ageing is caused in part by TGF-β, which reduces the subcutaneous fat that gives skin a pleasant appearance and texture. TGF-β does this by blocking the conversion of dermal fibroblasts into fat cells; with fewer fat cells underneath to provide support, the skin becomes saggy and wrinkled. Subcutaneous fat also produces cathelicidin, which is a peptide that fights bacterial infections.[109][110]
Damage-related factors
- DNA damage theory of ageing: DNA damage is thought to be the common basis of both cancer and ageing, and it has been argued that intrinsic causes of DNA damage are the most important drivers of ageing.[111][112][113] Genetic damage (aberrant structural alterations of the DNA), mutations (changes in the DNA sequence), and epimutations (methylation of gene promoter regions or alterations of the DNA scaffolding which regulate gene expression), can cause abnormal gene expression. DNA damage causes the cells to stop dividing or induces apoptosis, often affecting stem cell pools and hence hindering regeneration. However, lifelong studies of mice suggest that most mutations happen during embryonic and childhood development, when cells divide often, as each cell division is a chance for errors in DNA replication.[114]
- Genetic instability: Dogs annually lose approximately 3.3% of the DNA in their heart muscle cells while humans lose approximately 0.6% of their heart muscle DNA each year. These numbers are close to the ratio of the maximum longevities of the two species (120 years vs. 20 years, a 6/1 ratio). The comparative percentage is also similar between the dog and human for yearly DNA loss in the brain and lymphocytes. As stated by lead author, Bernard L. Strehler, "... genetic damage (particularly gene loss) is almost certainly (or probably the) central cause of ageing."[115]
- Accumulation of waste:
- A buildup of waste products in cells presumably interferes with metabolism. For example, a waste product called lipofuscin is formed by a complex reaction in cells that binds fat to proteins. This waste accumulates in the cells as small granules, which increase in size as a person ages.[116]
- The hallmark of ageing yeast cells appears to be overproduction of certain proteins.[57]
- Autophagy induction can enhance clearance of toxic intracellular waste associated with neurodegenerative diseases and has been comprehensively demonstrated to improve lifespan in yeast, worms, flies, rodents and primates. The situation, however, has been complicated by the identification that autophagy up-regulation can also occur during ageing.[117] Autophagy is enhanced in obese mice by caloric restriction, exercise, and a low fat diet (but in these mice is evidently not related with the activation of AMP-activated protein kinase, see above).[118]
- Wear-and-tear theory: The very general idea that changes associated with ageing are the result of chance damage that accumulates over time.[61]
- Accumulation of errors: The idea that ageing results from chance events that escape proof reading mechanisms, which gradually damages the genetic code.
- Heterochromatin loss, model of ageing.[119][120][121]
- Transposable elements in genome disintegration as the primary role in the mechanism of ageing.[122][123][124]
- Cross-linkage: The idea that ageing results from accumulation of cross-linked compounds that interfere with normal cell function.[99][125]
- Studies of mtDNA mutator mice have shown that increased levels of somatic mtDNA mutations directly can cause a variety of ageing phenotypes. The authors propose that mtDNA mutations lead to respiratory-chain-deficient cells and thence to apoptosis and cell loss. They cast doubt experimentally however on the common assumption that mitochondrial mutations and dysfunction lead to increased generation of reactive oxygen species (ROS).[126]
- Free-radical theory: Damage by free radicals, or more generally reactive oxygen species or oxidative stress, create damage that may give rise to the symptoms we recognise as ageing.[99][127] Michael Ristow's group has provided evidence that the effect of calorie restriction may be due to increased formation of free radicals within the mitochondria, causing a secondary induction of increased antioxidant defence capacity.[128]
- Mitochondrial theory of ageing: free radicals produced by mitochondrial activity damage cellular components, leading to ageing.
- DNA oxidation and caloric restriction: Caloric restriction reduces 8-OH-dG DNA damage in organs of ageing rats and mice.[129][130] Thus, reduction of oxidative DNA damage is associated with a slower rate of ageing and increased lifespan.[131]
Prevention and delay
Lifestyle
Caloric restriction substantially affects lifespan in many animals, including the ability to delay or prevent many age-related diseases.[132] Typically, this involves caloric intake of 60–70% of what an ad libitum animal would consume, while still maintaining proper nutrient intake.[132] In rodents, this has been shown to increase lifespan by up to 50%;[133] similar effects occur for yeast and Drosophila.[132] No lifespan data exist for humans on a calorie-restricted diet,[92] but several reports support protection from age-related diseases.[134][135] Two major ongoing studies on rhesus monkeys initially revealed disparate results; while one study, by the University of Wisconsin, showed that caloric restriction does extend lifespan,[136] the second study, by the National Institute on Aging (NIA), found no effects of caloric restriction on longevity.[137] Both studies nevertheless showed improvement in a number of health parameters. Notwithstanding the similarly low calorie intake, the diet composition differed between the two studies (notably a high sucrose content in the Wisconsin study), and the monkeys have different origins (India, China), initially suggesting that genetics and dietary composition, not merely a decrease in calories, are factors in longevity.[92] However, in a comparative analysis in 2014, the Wisconsin researchers found that the allegedly non-starved NIA control monkeys in fact are moderately underweight when compared with other monkey populations, and argued this was due to the NIA's apportioned feeding protocol in contrast to Wisconsin's truly unrestricted ad libitum feeding protocol.[138] They conclude that moderate calorie restriction rather than extreme calorie restriction is sufficient to produce the observed health and longevity benefits in the studied rhesus monkeys.[139]
In his book How and Why We Age, Hayflick says that caloric restriction may not be effective in humans, citing data from the Baltimore Longitudinal Study of Aging which shows that being thin does not favour longevity.[140] However, there may be confounders, e.g. smoking reduces both appetite and lifespan. Similarly, it is sometimes claimed that moderate obesity in later life may improve survival, but newer research has identified confounding factors such as weight loss due to terminal disease. Once these factors are accounted for, the optimal body weight above age 65 corresponds to a leaner body mass index of 23 to 27.[141]
Alternatively, the benefits of dietary restriction can also be found by changing the macro nutrient profile to reduce protein intake without any changes to calorie level, resulting in similar increases in longevity.[142][143] Dietary protein restriction not only inhibits mTOR activity but also IGF-1, two mechanisms implicated in ageing.[89] Specifically, reducing leucine intake is sufficient to inhibit mTOR activity, achievable through reducing animal food consumption.[144][145]
The Mediterranean diet is credited with lowering the risk of heart disease and early death.[146][147] The major contributors to mortality risk reduction appear to be a higher consumption of vegetables, fish, fruits, nuts and monounsaturated fatty acids, i.e., olive oil.[148]
The amount of sleep has an impact on mortality. People who live the longest report sleeping for six to seven hours each night.[149][150] Lack of sleep (<5 hours) more than doubles the risk of death from cardiovascular disease, but too much sleep (>9 hours) is associated with a doubling of the risk of death, though not primarily from cardiovascular disease.[151] Sleeping more than 7 to 8 hours per day has been consistently associated with increased mortality, though the cause is probably other factors such as depression and socioeconomic status, which would correlate statistically.[152] Sleep monitoring of hunter-gatherer tribes from Africa and from South America has shown similar sleep patterns across continents: their average sleeping duration is 6.4 hours (with a summer/winter difference of 1 hour), afternoon naps (siestas) are uncommon, and insomnia is very rare (tenfold less than in industrial societies).[153]
Physical exercise may increase life expectancy.[154] People who participate in moderate to high levels of physical exercise have a lower mortality rate compared to individuals who are not physically active.[155] Moderate levels of exercise have been correlated with preventing ageing and improving quality of life by reducing inflammatory potential.[156] The majority of the benefits from exercise are achieved with around 3500 metabolic equivalent (MET) minutes per week.[157] For example, climbing stairs 10 minutes, vacuuming 15 minutes, gardening 20 minutes, running 20 minutes, and walking or bicycling for 25 minutes on a daily basis would together achieve about 3000 MET minutes a week.[157] Other research seems to suggest a relationship between regular physical exercise and cognitive functioning in old age.[158]
Avoidance of chronic stress (as opposed to acute stress) is associated with a slower loss of telomeres in most but not all studies,[159][160] and with decreased cortisol levels. A chronically high cortisol level compromises the immune system, causes cardiac damage/arterosclerosis and is associated with facial ageing, and the latter in turn is a marker for increased morbidity and mortality.[161][162] A meta-analysis shows that loneliness carries a higher mortality risk than smoking.[163] Stress can be countered by social connection, spirituality, and (for men more clearly than for women) married life, all of which are associated with longevity.[164][165][166][167]
Medical intervention
The following drugs and interventions have been shown to slow or reverse the biological effects of ageing in animal models, but none has yet been proven to do so in humans.
Evidence in both animals and humans suggests that resveratrol may be a caloric restriction mimetic.[168]
As of 2015, metformin was under study for its potential effect on slowing ageing in the worm C.elegans and the cricket.[169] Its effect on otherwise healthy humans is unknown.[169]
Rapamycin was first shown to extend lifespan in eukaryotes in 2006 by Powers et al. who showed a dose-responsive effect of rapamycin on lifespan extension in yeast cells.[170] In a 2009 study, the lifespans of mice fed rapamycin were increased between 28 and 38% from the beginning of treatment, or 9 to 14% in total increased maximum lifespan. Of particular note, the treatment began in mice aged 20 months, the equivalent of 60 human years.[171] Rapamycin has subsequently been shown to extend mouse lifespan in several separate experiments,[172][173] and is now being tested for this purpose in nonhuman primates (the marmoset monkey).[174]
Cancer geneticist Ronald A. DePinho and his colleagues published research on mice where telomerase activity was first genetically removed. Then, after the mice had prematurely aged, they restored telomerase activity by reactivating the telomerase gene. As a result, the mice were rejuvenated: Shrivelled testes grew back to normal and the animals regained their fertility. Other organs, such as the spleen, liver, intestines and brain, recuperated from their degenerated state. "[The finding] offers the possibility that normal human ageing could be slowed by reawakening the enzyme in cells where it has stopped working" says Ronald DePinho. However, activating telomerase in humans could potentially encourage the growth of tumours.[175]
Most known genetic interventions in C. elegans increase lifespan by 1.5 to 2.5-fold. As of 2009, the record for lifespan extension in C. elegans is a single-gene mutation which increases adult survival by tenfold.[59] The strong conservation of some of the mechanisms of ageing discovered in model organisms imply that they may be useful in the enhancement of human survival. However, the benefits may not be proportional; longevity gains are typically greater in C. elegans than fruit flies, and greater in fruit flies than in mammals. One explanation for this is that mammals, being much longer-lived, already have many traits which promote lifespan.[59]
Research projects and prizes
Some research effort is directed to slow ageing and extend healthy lifespan.[176][177][178] In 1993, the Established populations for epidemiologic studies of the elderly,[179] also known as the Yale Health and Aging Study, showed the importance of physical activity and argued against negative stereotypes concerning old age.
The US National Institute on Aging currently funds an intervention testing programme, whereby investigators nominate compounds (based on specific molecular ageing theories) to have evaluated with respect to their effects on lifespan and age-related biomarkers in outbred mice.[180] Previous age-related testing in mammals has proved largely irreproducible, because of small numbers of animals and lax mouse husbandry conditions. The intervention testing programme aims to address this by conducting parallel experiments at three internationally recognised mouse ageing-centres, the Barshop Institute at UTHSCSA, the University of Michigan at Ann Arbor and the Jackson Laboratory.
Several companies and organisations, such as Google Calico, Human Longevity, Craig Venter, Gero,[181] SENS Research Foundation, and Science for Life Extension in Russia,[182] declared stopping or delaying ageing as their goal.
Prizes for extending lifespan and slowing ageing in mammals exist. The Methuselah Foundation offers the Mprize. Recently, the $1 Million Palo Alto Longevity Prize was launched. It is a research incentive prize to encourage teams from all over the world to compete in an all-out effort to "hack the code" that regulates our health and lifespan. It was founded by Joon Yun.[183][184][185][186][187]
Society and culture
| Look up quadragenarian, quinquagenarian, sexagenarian, septuagenarian, octogenarian, or nonagenarian in Wiktionary, the free dictionary. |

Different cultures express age in different ways. The age of an adult human is commonly measured in whole years since the day of birth. Arbitrary divisions set to mark periods of life may include: juvenile (via infancy, childhood, preadolescence, adolescence), early adulthood, middle adulthood, and late adulthood. More casual terms may include "teenagers", "tweens", "twentysomething", "thirtysomething", etc. as well as "denarian", "vicenarian", "tricenarian", "quadragenarian", etc.
Most legal systems define a specific age for when an individual is allowed or obliged to do particular activities. These age specifications include voting age, drinking age, age of consent, age of majority, age of criminal responsibility, marriageable age, age of candidacy, and mandatory retirement age. Admission to a movie for instance, may depend on age according to a motion picture rating system. A bus fare might be discounted for the young or old. Each nation, government and non-governmental organisation has different ways of classifying age. In other words, chronological ageing may be distinguished from "social ageing" (cultural age-expectations of how people should act as they grow older) and "biological ageing" (an organism's physical state as it ages).[188]
Ageism cost the United States $63 billion in one year according to a Yale School of Public Health study.[189] In a UNFPA report about ageing in the 21st century, it highlighted the need to "Develop a new rights-based culture of ageing and a change of mindset and societal attitudes towards ageing and older persons, from welfare recipients to active, contributing members of society".[190] UNFPA said that this "requires, among others, working towards the development of international human rights instruments and their translation into national laws and regulations and affirmative measures that challenge age discrimination and recognise older people as autonomous subjects".[190] Older people's music participation contributes to the maintenance of interpersonal relationships and promoting successful ageing.[191] At the same time, older persons can make contributions to society including caregiving and volunteering. For example, "A study of Bolivian migrants who [had] moved to Spain found that 69% left their children at home, usually with grandparents. In rural China, grandparents care for 38% of children aged under five whose parents have gone to work in cities."[190]
Economics
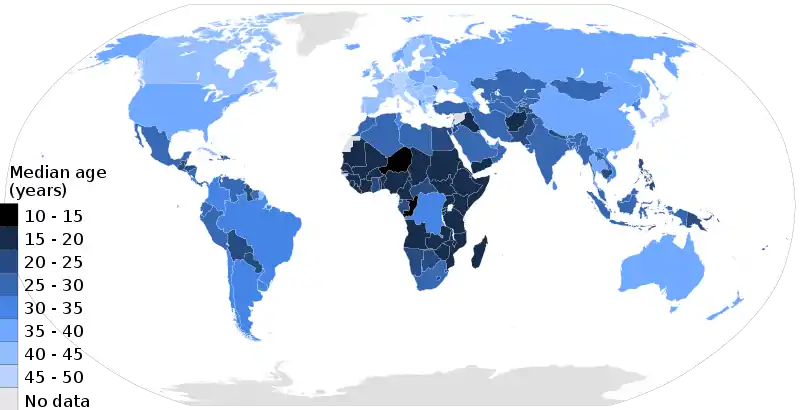
Population ageing is the increase in the number and proportion of older people in society. Population ageing has three possible causes: migration, longer life expectancy (decreased death rate) and decreased birth rate. Ageing has a significant impact on society. Young people tend to have fewer legal privileges (if they are below the age of majority), they are more likely to push for political and social change, to develop and adopt new technologies, and to need education. Older people have different requirements from society and government, and frequently have differing values as well, such as for property and pension rights.[192]
In the 21st century, one of the most significant population trends is ageing.[193] Currently, over 11% of the world's current population are people aged 60 and older and the United Nations Population Fund (UNFPA) estimates that by 2050 that number will rise to approximately 22%.[190] Ageing has occurred due to development which has enabled better nutrition, sanitation, health care, education and economic well-being. Consequently, fertility rates have continued to decline and life expectancy has risen. Life expectancy at birth is over 80 now in 33 countries. Ageing is a "global phenomenon", that is occurring fastest in developing countries, including those with large youth populations, and poses social and economic challenges to the work which can be overcome with "the right set of policies to equip individuals, families and societies to address these challenges and to reap its benefits".[194]
As life expectancy rises and birth rates decline in developed countries, the median age rises accordingly. According to the United Nations, this process is taking place in nearly every country in the world.[195] A rising median age can have significant social and economic implications, as the workforce gets progressively older and the number of old workers and retirees grows relative to the number of young workers. Older people generally incur more health-related costs than do younger people in the workplace and can also cost more in worker's compensation and pension liabilities.[196] In most developed countries an older workforce is somewhat inevitable. In the United States for instance, the Bureau of Labor Statistics estimates that one in four American workers will be 55 or older by 2020.[196]
Among the most urgent concerns of older persons worldwide is income security. This poses challenges for governments with ageing populations to ensure investments in pension systems continues in order to provide economic independence and reduce poverty in old age. These challenges vary for developing and developed countries. UNFPA stated that, "Sustainability of these systems is of particular concern, particularly in developed countries, while social protection and old-age pension coverage remain a challenge for developing countries, where a large proportion of the labour force is found in the informal sector."[190]
The global economic crisis has increased financial pressure to ensure economic security and access to health care in old age. In order to elevate this pressure "social protection floors must be implemented in order to guarantee income security and access to essential health and social services for all older persons and provide a safety net that contributes to the postponement of disability and prevention of impoverishment in old age".[190]
It has been argued that population ageing has undermined economic development.[197] Evidence suggests that pensions, while making a difference to the well-being of older persons, also benefit entire families especially in times of crisis when there may be a shortage or loss of employment within households. A study by the Australian Government in 2003 estimated that "women between the ages of 65 and 74 years contribute A$16 billion per year in unpaid caregiving and voluntary work. Similarly, men in the same age group contributed A$10 billion per year."[190]
Due to increasing share of the elderly in the population, health care expenditures will continue to grow relative to the economy in coming decades. This has been considered as a negative phenomenon and effective strategies like labour productivity enhancement should be considered to deal with negative consequences of ageing.[198]
Sociology
.png.webp)
In the field of sociology and mental health, ageing is seen in five different views: ageing as maturity, ageing as decline, ageing as a life-cycle event, ageing as generation, and ageing as survival.[199] Positive correlates with ageing often include economics, employment, marriage, children, education, and sense of control, as well as many others. The social science of ageing includes disengagement theory, activity theory, selectivity theory, and continuity theory. Retirement, a common transition faced by the elderly, may have both positive and negative consequences.[200] As cyborgs currently are on the rise some theorists argue there is a need to develop new definitions of ageing and for instance a bio-techno-social definition of ageing has been suggested.[201]
There is a current debate as to whether or not the pursuit of longevity and the postponement of senescence are cost-effective health care goals given finite health care resources. Because of the accumulated infirmities of old age, bioethicist Ezekiel Emanuel, opines that the pursuit of longevity via the compression of morbidity hypothesis is a "fantasy" and that human life is not worth living after age 75; longevity then should not be a goal of health care policy.[202] This opinion has been contested by neurosurgeon and medical ethicist Miguel Faria, who states that life can be worthwhile during old age, and that longevity should be pursued in association with the attainment of quality of life.[203] Faria claims that postponement of senescence as well as happiness and wisdom can be attained in old age in a large proportion of those who lead healthy lifestyles and remain intellectually active.[204]
Health care demand
With age inevitable biological changes occur that increase the risk of illness and disability. UNFPA states that,[194]
"A life-cycle approach to health care – one that starts early, continues through the reproductive years and lasts into old age – is essential for the physical and emotional well-being of older persons, and, indeed, all people. Public policies and programmes should additionally address the needs of older impoverished people who cannot afford health care."
Many societies in Western Europe and Japan have ageing populations. While the effects on society are complex, there is a concern about the impact on health care demand. The large number of suggestions in the literature for specific interventions to cope with the expected increase in demand for long-term care in ageing societies can be organised under four headings: improve system performance; redesign service delivery; support informal caregivers; and shift demographic parameters.[205]
However, the annual growth in national health spending is not mainly due to increasing demand from ageing populations, but rather has been driven by rising incomes, costly new medical technology, a shortage of health care workers and informational asymmetries between providers and patients.[206] A number of health problems become more prevalent as people get older. These include mental health problems as well as physical health problems, especially dementia.
It has been estimated that population ageing only explains 0.2 percentage points of the annual growth rate in medical spending of 4.3% since 1970. In addition, certain reforms to the Medicare system in the United States decreased elderly spending on home health care by 12.5% per year between 1996 and 2000.[207]
Self-perception of ageing
The beauty standards are constantly evolving over decades due to increased perception of esthetics.[208] Because of that, the cosmeceutical industry is expanding and gradually becoming a part of many people's personal care routine. Cosmeceutical is currently the fastest growing beauty industry, with more than $42 billion in 2018.[209]
Cryptomphalus aspersa secretion (or brown garden snail secretion) has been found to have antioxidant properties, increase skin cell proliferation, as well as increase extracellular proteins such as collagen and fibronectin (important proteins for cell proliferation).[210]
Positive self-perceptions of ageing are associated with better mental and physical health and well-being.[211]
Positive self-perception of health has been correlated with higher well-being and reduced mortality in the elderly.[212][213] Various reasons have been proposed for this association; people who are objectively healthy may naturally rate their health better than that of their ill counterparts, though this link has been observed even in studies which have controlled for socioeconomic status, psychological functioning and health status.[214] This finding is generally stronger for men than women,[213] though this relationship is not universal across all studies and may only be true in some circumstances.[214]
As people age, subjective health remains relatively stable, even though objective health worsens.[215] In fact, perceived health improves with age when objective health is controlled in the equation.[216] This phenomenon is known as the "paradox of ageing". This may be a result of social comparison;[217] for instance, the older people get, the more they may consider themselves in better health than their same-aged peers.[218] Elderly people often associate their functional and physical decline with the normal ageing process.[219][220]
Successful ageing
The concept of successful ageing can be traced back to the 1950s and was popularised in the 1980s. Traditional definitions of successful ageing have emphasised absence of physical and cognitive disabilities.[221] In their 1987 article, Rowe and Kahn characterised successful ageing as involving three components: a) freedom from disease and disability, b) high cognitive and physical functioning, and c) social and productive engagement.[222]
Cultural references
The ancient Greek dramatist Euripides (5th century BC) describes the multiple-headed mythological monster Hydra as having a regenerative capacity which makes it immortal, which is the historical background to the name of the biological genus Hydra. The Book of Job (c. 6th century BC) describes human lifespan as inherently limited and makes a comparison with the innate immortality that a felled tree may have when undergoing vegetative regeneration.[223]
See also
- Ageing brain
- Ageing movement control
- Ageing of Europe
- Ageing studies
- Anti-ageing movement
- Biodemography of human longevity
- Biogerontology
- Biological immortality
- Biomarkers of ageing
- Clinical geropsychology
- Death
- Epigenetic clock
- Evolution of ageing
- Genetics of ageing
- Gerontology
- Gerascophobia
- List of life extension-related topics
- Longevity
- Mitochondrial theory of ageing
- Neuroscience of ageing
- Old age
- Population ageing
- Progeria
- Stem cell theory of ageing
- Supercentenarian
- Transgenerational design
References
- Bowen RL, Atwood CS (2004). "Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones". Gerontology. 50 (5): 265–90. doi:10.1159/000079125. PMID 15331856. S2CID 18109386.
- Ghosh, Shampa; Sinha, Jitendra Kumar; Raghunath, Manchala (2016). "Epigenomic maintenance through dietary intervention can facilitate DNA repair process to slow down the progress of premature aging". IUBMB Life. 68 (9): 717–721. doi:10.1002/iub.1532. ISSN 1521-6551. PMID 27364681.
- Ghosh, Shampa; Sinha, Jitendra Kumar; Raghunath, Manchala (May 2019). "'Obesageing': Linking obesity & ageing". The Indian Journal of Medical Research. 149 (5): 610–615. doi:10.4103/ijmr.IJMR_2120_18. ISSN 0971-5916. PMC 6702696. PMID 31417028.
- Salvestrini, Valentina; Sell, Christian; Lorenzini, Antonello (3 May 2019). "Obesity May Accelerate the Aging Process". Frontiers in Endocrinology. 10: 266. doi:10.3389/fendo.2019.00266. ISSN 1664-2392. PMC 6509231. PMID 31130916.
- McDonald, Roger B. (7 June 2019), "Basic Concepts in the Biology of Aging", Biology of Aging, Garland Science, pp. 1–36, doi:10.1201/9780429030642-1, ISBN 978-0-429-03064-2
- Mortimer RK, Johnston JR (June 1959). "Life span of individual yeast cells". Nature. 183 (4677): 1751–2. Bibcode:1959Natur.183.1751M. doi:10.1038/1831751a0. hdl:2027/mdp.39015078535278. PMID 13666896. S2CID 4149521.
- Nutman AP, Bennett VC, Friend CR, Van Kranendonk MJ, Chivas AR (September 2016). "Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures". Nature (Submitted manuscript). 537 (7621): 535–538. Bibcode:2016Natur.537..535N. doi:10.1038/nature19355. PMID 27580034. S2CID 205250494.
- Rose MR (1991). Evolutionary Biology of Aging. New York: Oxford University Press.
- Partridge L, Barton NH (March 1993). "Optimality, mutation and the evolution of ageing". Nature. 362 (6418): 305–11. Bibcode:1993Natur.362..305P. doi:10.1038/362305a0. PMID 8455716. S2CID 4330925.
- Williams GC (1957). "Pleiotropy, Natural Selection, and the Evolution of Senescence". Evolution. 11 (4): 398–411. doi:10.2307/2406060. JSTOR 2406060. Lay summary.
- Stewart EJ, Madden R, Paul G, Taddei F (February 2005). "Aging and death in an organism that reproduces by morphologically symmetric division". PLOS Biology. 3 (2): e45. doi:10.1371/journal.pbio.0030045. PMC 546039. PMID 15685293.
- Pereira-Smith OM, Ning Y (1992). "Molecular genetic studies of cellular senescence". Experimental Gerontology. 27 (5–6): 519–22. doi:10.1016/0531-5565(92)90006-L. PMID 1426085. S2CID 27839420.
- Forster P, Hohoff C, Dunkelmann B, Schürenkamp M, Pfeiffer H, Neuhuber F, Brinkmann B (March 2015). "Elevated germline mutation rate in teenage fathers". Proceedings. Biological Sciences. 282 (1803): 20142898. doi:10.1098/rspb.2014.2898. PMC 4345458. PMID 25694621.
- Wakayama S, Kohda T, Obokata H, Tokoro M, Li C, Terashita Y, et al. (March 2013). "Successful serial recloning in the mouse over multiple generations". Cell Stem Cell. 12 (3): 293–7. doi:10.1016/j.stem.2013.01.005. PMID 23472871.
- Moss S (July 2013). "Big ears: they really do grow as we age". The Guardian. MeshID:D000375; OMIM:502000. Retrieved 9 September 2016.
- Gerasimov IG, Ignatov DY (2004). "Age Dynamics of Body Mass and Human Lifespan". Journal of Evolutionary Biochemistry and Physiology. 40 (3): 343–349. doi:10.1023/B:JOEY.0000042639.72529.e1. S2CID 9070790.
- Rodríguez Valiente A, Trinidad A, García Berrocal JR, Górriz C, Ramírez Camacho R (August 2014). "Extended high-frequency (9-20 kHz) audiometry reference thresholds in 645 healthy subjects". International Journal of Audiology. 53 (8): 531–45. doi:10.3109/14992027.2014.893375. PMID 24749665. S2CID 30960789.
- Thurstan SA, Gibbs NK, Langton AK, Griffiths CE, Watson RE, Sherratt MJ (April 2012). "Chemical consequences of cutaneous photoageing". Chemistry Central Journal. 6 (1): 34. doi:10.1186/1752-153X-6-34. PMC 3410765. PMID 22534143.
- pmhdev (25 March 2015). "Infertility: Overview". Institute for Quality and Efficiency in Health Care (IQWiG) – via www.ncbi.nlm.nih.gov. Cite journal requires
|journal=(help) - Ryall JG, Schertzer JD, Lynch GS (August 2008). "Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness". Biogerontology. 9 (4): 213–28. doi:10.1007/s10522-008-9131-0. PMID 18299960. S2CID 8576449.
- Betik AC, Hepple RT (February 2008). "Determinants of VO2 max decline with aging: an integrated perspective". Applied Physiology, Nutrition, and Metabolism. 33 (1): 130–40. doi:10.1139/H07-174. PMID 18347663. S2CID 24468921.
- Ranganathan, Vinoth K.; Siemionow, Vlodek; Sahgal, Vinod; Yue, Guang H. (November 2001). "Effects of Aging on Hand Function". Journal of the American Geriatrics Society. 49 (11): 1478–1484. doi:10.1046/j.1532-5415.2001.4911240.x. ISSN 0002-8614. PMID 11890586. S2CID 22988219.
- "Facts About Presbyopia". Last Reviewed October 2010: National Eye Institute. Retrieved 11 September 2016.CS1 maint: location (link)
- Weale RA (2003). "Epidemiology of refractive errors and presbyopia". Survey of Ophthalmology. 48 (5): 515–43. doi:10.1016/S0039-6257(03)00086-9. PMID 14499819.
- Truscott RJ (February 2009). "Presbyopia. Emerging from a blur towards an understanding of the molecular basis for this most common eye condition". Experimental Eye Research. 88 (2): 241–7. doi:10.1016/j.exer.2008.07.003. PMID 18675268.
- Pathai S, Shiels PG, Lawn SD, Cook C, Gilbert C (March 2013). "The eye as a model of ageing in translational research--molecular, epigenetic and clinical aspects". Ageing Research Reviews. 12 (2): 490–508. doi:10.1016/j.arr.2012.11.002. PMID 23274270. S2CID 26015190.
- Pandhi D, Khanna D (2013). "Premature graying of hair". Indian Journal of Dermatology, Venereology and Leprology. 79 (5): 641–53. doi:10.4103/0378-6323.116733. PMID 23974581.
- Hamilton JB (March 1951). "Patterned loss of hair in man; types and incidence". Annals of the New York Academy of Sciences. 53 (3): 708–28. Bibcode:1951NYASA..53..708H. doi:10.1111/j.1749-6632.1951.tb31971.x. PMID 14819896. S2CID 32685699.
- Vary JC (November 2015). "Selected Disorders of Skin Appendages--Acne, Alopecia, Hyperhidrosis". The Medical Clinics of North America. 99 (6): 1195–211. doi:10.1016/j.mcna.2015.07.003. PMID 26476248.
- Morabia A, Costanza MC (December 1998). "International variability in ages at menarche, first livebirth, and menopause. World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives". American Journal of Epidemiology. 148 (12): 1195–205. doi:10.1093/oxfordjournals.aje.a009609. PMID 9867266.
- Thomas E, Peat G, Croft P (February 2014). "Defining and mapping the person with osteoarthritis for population studies and public health". Rheumatology. 53 (2): 338–45. doi:10.1093/rheumatology/ket346. PMC 3894672. PMID 24173433.
- "Hearing Loss and Older Adults" (Last Updated 3 June 2016). National Institute on Deafness and Other Communication Disorders. 26 January 2016. Retrieved 11 September 2016.
- Rubel EW, Furrer SA, Stone JS (March 2013). "A brief history of hair cell regeneration research and speculations on the future". Hearing Research. 297: 42–51. doi:10.1016/j.heares.2012.12.014. PMC 3657556. PMID 23321648.
- "Facts About Cataract". September 2015. Retrieved 14 August 2016.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. (March 2001). "Frailty in older adults: evidence for a phenotype". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 56 (3): M146-56. CiteSeerX 10.1.1.456.139. doi:10.1093/gerona/56.3.m146. PMID 11253156.
- Percentage derived from Table 2 in Fried et al. 2001
- Wang JC, Bennett M (July 2012). "Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence". Circulation Research. 111 (2): 245–59. doi:10.1161/CIRCRESAHA.111.261388. PMID 22773427.
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S (February 2016). "Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease". Circulation Research. 118 (4): 535–46. doi:10.1161/CIRCRESAHA.115.307611. PMID 26892956.
- "Does Human Life Span Really Have a Limit?". WebMD. 28 June 2018.
- Zimmer, Carl (5 October 2016). "What's the Longest Humans Can Live? 115 Years, New Study Says". The New York Times. Retrieved 6 October 2016.
- Dong X, Milholland B, Vijg J (October 2016). "Evidence for a limit to human lifespan". Nature. 538 (7624): 257–259. Bibcode:2016Natur.538..257D. doi:10.1038/nature19793. PMID 27706136. S2CID 3623127.
- Larson EB, Yaffe K, Langa KM (December 2013). "New insights into the dementia epidemic". The New England Journal of Medicine. 369 (24): 2275–7. doi:10.1056/nejmp1311405. PMC 4130738. PMID 24283198.
- Umphred D (2012). Neurological rehabilitation (6th ed.). St. Louis, MO: Elsevier Mosby. p. 838. ISBN 978-0-323-07586-2.
- Schaie, K. Warner v (2005). Developmental Influences on Adult Intelligence. doi:10.1093/acprof:oso/9780195156737.001.0001. ISBN 978-0-19-515673-7.
- Stuart-Hamilton I (2006). The Psychology of Ageing: An Introduction. London: Jessica Kingsley Publishers. ISBN 978-1-84310-426-1.
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B (July 2003). "Marked loss of myelinated nerve fibers in the human brain with age". The Journal of Comparative Neurology. 462 (2): 144–52. doi:10.1002/cne.10714. PMID 12794739. S2CID 35293796.
- Peters A (1 January 2007). "The Effects of Normal Aging on Nerve Fibers and Neuroglia in the Central Nervous System". In Riddle DR (ed.). Brain Aging: Models, Methods, and Mechanisms. Frontiers in Neuroscience. CRC Press/Taylor & Francis. ISBN 978-0-8493-3818-2. PMID 21204349.
- Worrall L, Hickson LM (2003). "Theoretical foundations of communication disability in aging". In Worrall L, Hickson LM (eds.). Communication disability in aging: from prevention to intervention. Clifton Park, NY: Delmar Learning. pp. 32–33.
- Lys R, Belanger E, Phillips SP (April 2019). "Improved mood despite worsening physical health in older adults: Findings from the International Mobility in Aging Study (IMIAS)". PLOS ONE. 14 (4): e0214988. Bibcode:2019PLoSO..1414988L. doi:10.1371/journal.pone.0214988. PMC 6453471. PMID 30958861.
- Mehta S (September 2015). "Age-Related Macular Degeneration". Primary Care. 42 (3): 377–91. doi:10.1016/j.pop.2015.05.009. PMID 26319344.
- Nussbaum JF, Thompson TL, Robinson JD (1989). "Barriers to conversation". In Nussbaum JF, Thompson TL, Robinson JD (eds.). Communication and aging. New York: Harper & Row. pp. 234–53.
- Belikov AV (January 2019). "Age-related diseases as vicious cycles". Ageing Research Reviews. 49: 11–26. doi:10.1016/j.arr.2018.11.002. PMID 30458244. S2CID 53567141.
- De Grey AD (2007). "Life Span Extension Research and Public Debate: Societal Considerations". Studies in Ethics, Law, and Technology. 1. CiteSeerX 10.1.1.395.745. doi:10.2202/1941-6008.1011.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (May 2006). "Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data". Lancet. 367 (9524): 1747–57. doi:10.1016/S0140-6736(06)68770-9. PMID 16731270. S2CID 22609505.
- Brunet Lab: Molecular Mechanisms of Longevity and Age Related Diseases. Stanford.edu. Retrieved on 11 April 2012.
- Janssens GE, Meinema AC, González J, Wolters JC, Schmidt A, Guryev V, et al. (December 2015). "Protein biogenesis machinery is a driver of replicative aging in yeast". eLife. 4: e08527. doi:10.7554/eLife.08527. PMC 4718733. PMID 26422514.
- Wilkinson DS, Taylor RC, Dillin A (2012). "Analysis of Aging in Caenorhabditis elegans". In Rothman JH, Singson A (eds.). Caenorhabditis Elegans: Cell Biology and Physiology. Academic Press. pp. 353–81. ISBN 978-0-12-394620-1.
- Shmookler Reis RJ, Bharill P, Tazearslan C, Ayyadevara S (October 2009). "Extreme-longevity mutations orchestrate silencing of multiple signaling pathways". Biochimica et Biophysica Acta (BBA) - General Subjects. 1790 (10): 1075–83. doi:10.1016/j.bbagen.2009.05.011. PMC 2885961. PMID 19465083.
- "Mitochondrial Theory of Aging and Other Aging Theories". 1Vigor. Retrieved 4 October 2013.
- Jin K (October 2010). "Modern Biological Theories of Aging". Aging and Disease. 1 (2): 72–74. PMC 2995895. PMID 21132086.
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (June 2013). "The hallmarks of aging". Cell. 153 (6): 1194–217. doi:10.1016/j.cell.2013.05.039. PMC 3836174. PMID 23746838.
- Berdyshev GD, Korotaev GK, Boiarskikh GV, Vaniushin BF (2008). "Molecular Biology of Aging". Cell. Cold Spring Harbor. 96 (2): 347–62. doi:10.1016/s0092-8674(00)80567-x. ISBN 978-0-87969-824-9. PMID 9988222. S2CID 17724023.
- Taylor RC, Dillin A (May 2011). "Aging as an event of proteostasis collapse". Cold Spring Harbor Perspectives in Biology. 3 (5): a004440. doi:10.1101/cshperspect.a004440. PMC 3101847. PMID 21441594.
- Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T (December 2008). "Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana". Nature Genetics. 40 (12): 1489–92. doi:10.1038/ng.253. PMID 18997783. S2CID 13225884.
- Chesterton M (12 June 2017). "The oldest living thing on Earth". BBC News. Retrieved 16 September 2017.
- "Oldlist". Rocky Mountain Tree Ring Research. Retrieved 12 August 2016.
- Sosnowska D, Richardson C, Sonntag WE, Csiszar A, Ungvari Z, Ridgway I (December 2014). "A heart that beats for 500 years: age-related changes in cardiac proteasome activity, oxidative protein damage and expression of heat shock proteins, inflammatory factors, and mitochondrial complexes in Arctica islandica, the longest-living noncolonial animal". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 69 (12): 1448–61. doi:10.1093/gerona/glt201. PMC 4271020. PMID 24347613.
- Nielsen J, Hedeholm RB, Heinemeier J, Bushnell PG, Christiansen JS, Olsen J, et al. (August 2016). "Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus)". Science. 353 (6300): 702–4. Bibcode:2016Sci...353..702N. doi:10.1126/science.aaf1703. PMID 27516602. S2CID 206647043.
- Durkin A, Fisher CR, Cordes EE (August 2017). "Extreme longevity in a deep-sea vestimentiferan tubeworm and its implications for the evolution of life history strategies". Die Naturwissenschaften. 104 (7–8): 63. Bibcode:2017SciNa.104...63D. doi:10.1007/s00114-017-1479-z. PMID 28689349. S2CID 11287549.
- Timiras, Paola S. (2003) Physiological Basis of Ageing and Geriatrics. Informa Health Care. ISBN 0-8493-0948-4. p. 26.
- Silverman J (5 July 2007). "Is there a 400 pound lobster out there?". howstuffworks.
- Wallace DF (2005). Consider the Lobster and Other Essays. Little, Brown & Company. ISBN 978-0-316-15611-0.
- Guerin JC (June 2004). "Emerging area of aging research: long-lived animals with "negligible senescence"". Annals of the New York Academy of Sciences. 1019 (1): 518–20. Bibcode:2004NYASA1019..518G. doi:10.1196/annals.1297.096. PMID 15247078. S2CID 6418634.
- Bartke A (January 2011). "Single-gene mutations and healthy ageing in mammals". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 366 (1561): 28–34. doi:10.1098/rstb.2010.0281. PMC 3001310. PMID 21115527.
- "What 3 Things Can I Do to Extend the Length of My Life?". Time. Retrieved 15 November 2018.
- Berdyshev GD, Korotaev GK, Boiarskikh GV, Vaniushin BF (1967). "[Nucleotide composition of DNA and RNA from somatic tissues of humpback and its changes during spawning]". Biokhimiia (Moscow, Russia) (in Russian). 32 (5): 988–93. PMID 5628601.
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, et al. (January 2015). "DNA methylation age of blood predicts all-cause mortality in later life". Genome Biology. 16 (1): 25. doi:10.1186/s13059-015-0584-6. PMC 4350614. PMID 25633388.
- Christiansen L, Lenart A, Tan Q, Vaupel JW, Aviv A, McGue M, Christensen K (February 2016). "DNA methylation age is associated with mortality in a longitudinal Danish twin study". Aging Cell. 15 (1): 149–54. doi:10.1111/acel.12421. PMC 4717264. PMID 26594032.
- Horvath S, Pirazzini C, Bacalini MG, Gentilini D, Di Blasio AM, Delledonne M, et al. (December 2015). "Decreased epigenetic age of PBMCs from Italian semi-supercentenarians and their offspring". Aging. 7 (12): 1159–70. doi:10.18632/aging.100861. PMC 4712339. PMID 26678252.
- Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, et al. (December 2016). "In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming". Cell. 167 (7): 1719–1733.e12. doi:10.1016/j.cell.2016.11.052. PMC 5679279. PMID 27984723.
- Saey TJ (15 December 2016). "Proteins that reprogram cells can turn back mice's aging clock". Retrieved 19 December 2016.
- Callaway E (2016). "Destroying worn-out cells makes mice live longer". Nature. doi:10.1038/nature.2016.19287. S2CID 181078450. Retrieved 25 May 2019.
- Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, et al. (July 2016). "Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells". Aging. 8 (7): 1294–315. doi:10.18632/aging.100991. PMC 4993332. PMID 27391570.
- "Great Results". oisinbio.com. Retrieved 25 May 2019.
Oisín has shown as much as an 80% reduction in senescent cells in cell culture and significant reductions of senescent cell burden in naturally aged mice.
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, et al. (September 2008). "FOXO3A genotype is strongly associated with human longevity". Proceedings of the National Academy of Sciences of the United States of America. 105 (37): 13987–92. Bibcode:2008PNAS..10513987W. doi:10.1073/pnas.0801030105. PMC 2544566. PMID 18765803.
- Flachsbart F, Caliebe A, Kleindorp R, Blanché H, von Eller-Eberstein H, Nikolaus S, et al. (February 2009). "Association of FOXO3A variation with human longevity confirmed in German centenarians". Proceedings of the National Academy of Sciences of the United States of America. 106 (8): 2700–5. Bibcode:2009PNAS..106.2700F. doi:10.1073/pnas.0809594106. PMC 2650329. PMID 19196970.
- Ghosh HS, McBurney M, Robbins PD (February 2010). "SIRT1 negatively regulates the mammalian target of rapamycin". PLOS ONE. 5 (2): e9199. Bibcode:2010PLoSO...5.9199G. doi:10.1371/journal.pone.0009199. PMC 2821410. PMID 20169165.
- Fontana L, Partridge L, Longo VD (April 2010). "Extending healthy life span--from yeast to humans". Science. 328 (5976): 321–6. Bibcode:2010Sci...328..321F. doi:10.1126/science.1172539. PMC 3607354. PMID 20395504.
- Johnson SC, Rabinovitch PS, Kaeberlein M (January 2013). "mTOR is a key modulator of ageing and age-related disease". Nature. 493 (7432): 338–45. Bibcode:2013Natur.493..338J. doi:10.1038/nature11861. PMC 3687363. PMID 23325216.
- Halicka HD, Zhao H, Li J, Lee YS, Hsieh TC, Wu JM, Darzynkiewicz Z (December 2012). "Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling". Aging. 4 (12): 952–65. doi:10.18632/aging.100521. PMC 3615161. PMID 23363784.
- Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ (June 2013). "The GH/IGF-1 axis in ageing and longevity". Nature Reviews. Endocrinology. 9 (6): 366–376. doi:10.1038/nrendo.2013.67. PMC 4074016. PMID 23591370.
- Sun J, Kale SP, Childress AM, Pinswasdi C, Jazwinski SM (July 1994). "Divergent roles of RAS1 and RAS2 in yeast longevity". The Journal of Biological Chemistry. 269 (28): 18638–45. PMID 8034612.
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD (January 2008). "Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9". PLOS Genetics. 4 (1): e13. doi:10.1371/journal.pgen.0040013. PMC 2213705. PMID 18225956.
- "10-Fold Life Span Extension Reported". University of Southern California. Archived from the original on 4 March 2016. Retrieved 7 January 2016.
- Stibich, Mark (19 April 2009) Telomere Shortening – The Secret to Aging?. About.com
- Mikhelson VM, Gamaley IA (December 2012). "Telomere shortening is a sole mechanism of aging in mammals". Current Aging Science. 5 (3): 203–8. doi:10.2174/1874609811205030006. PMID 23387887.
- Hayflick, L. (1987) Origins of longevity. In Warner, H.R., Butler, R.N., Sprott, R.L. and Schneider, E.L. (eds), Modern Biological Theories of Aging. Raven Press, New York, pp. 21–34. ISBN 0-88167-310-2
- Bernstein C, Bernstein H. (1991) Aging, Sex, and DNA Repair. Academic Press, San Diego. ISBN 0-12-092860-4. pp. 314, 320, 326
- Hemann MT, Greider CW (November 2000). "Wild-derived inbred mouse strains have short telomeres". Nucleic Acids Research. 28 (22): 4474–8. doi:10.1093/nar/28.22.4474. PMC 113886. PMID 11071935.
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW (October 1997). "Telomere shortening and tumor formation by mouse cells lacking telomerase RNA". Cell. 91 (1): 25–34. doi:10.1016/S0092-8674(01)80006-4. PMID 9335332. S2CID 13366934.
- Kipling D, Cooke HJ (September 1990). "Hypervariable ultra-long telomeres in mice". Nature. 347 (6291): 400–2. Bibcode:1990Natur.347..400K. doi:10.1038/347400a0. PMID 2170845. S2CID 4358923.
- Nordfjäll K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G (February 2009). "The individual blood cell telomere attrition rate is telomere length dependent". PLOS Genetics. 5 (2): e1000375. doi:10.1371/journal.pgen.1000375. PMC 2633043. PMID 19214207.
- Nyström T (July 2003). "The free-radical hypothesis of aging goes prokaryotic". Cellular and Molecular Life Sciences. 60 (7): 1333–41. doi:10.1007/s00018-003-2310-X. PMID 12943222. S2CID 8406111.
- Kang HJ, Feng Z, Sun Y, Atwal G, Murphy ME, Rebbeck TR, et al. (June 2009). "Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans". Proceedings of the National Academy of Sciences of the United States of America. 106 (24): 9761–6. Bibcode:2009PNAS..106.9761K. doi:10.1073/pnas.0904280106. PMC 2700980. PMID 19470478.
- Smith KR, Hanson HA, Mineau GP, Buys SS (April 2012). "Effects of BRCA1 and BRCA2 mutations on female fertility". Proceedings. Biological Sciences. 279 (1732): 1389–95. doi:10.1098/rspb.2011.1697. PMC 3282366. PMID 21993507.
- Atwood CS, Bowen RL (2011). "The reproductive-cell cycle theory of aging: an update". Experimental Gerontology. 46 (2–3): 100–7. doi:10.1016/j.exger.2010.09.007. PMID 20851172. S2CID 20998909.
- Mair W, Steffen KK, Dillin A (September 2011). "SIP-ing the elixir of youth". Cell. 146 (6): 859–60. doi:10.1016/j.cell.2011.08.026. PMID 21925309.
- Galindo, Yadira (26 December 2018). "UC San Diego Researchers Identify How Skin Ages, Loses Fat and Immunity" (Press release). University of California San Diego.
- Zhang LJ, Chen SX, Guerrero-Juarez CF, Li F, Tong Y, Liang Y, et al. (January 2019). "Age-Related Loss of Innate Immune Antimicrobial Function of Dermal Fat Is Mediated by Transforming Growth Factor Beta". Immunity. 50 (1): 121–136.e5. doi:10.1016/j.immuni.2018.11.003. PMC 7191997. PMID 30594464.
- Gensler HL, Bernstein H (September 1981). "DNA damage as the primary cause of aging". The Quarterly Review of Biology. 56 (3): 279–303. doi:10.1086/412317. JSTOR 2826464. PMID 7031747. S2CID 20822805.
- Sinha JK, Ghosh S, Swain U, Giridharan NV, Raghunath M (June 2014). "Increased macromolecular damage due to oxidative stress in the neocortex and hippocampus of WNIN/Ob, a novel rat model of premature aging". Neuroscience. 269: 256–64. doi:10.1016/j.neuroscience.2014.03.040. PMID 24709042. S2CID 9934178.
- Freitas AA, de Magalhães JP (2011). "A review and appraisal of the DNA damage theory of ageing". Mutation Research. 728 (1–2): 12–22. doi:10.1016/j.mrrev.2011.05.001. PMID 21600302.
- Robert L, Labat-Robert J, Robert AM (August 2010). "Genetic, epigenetic and posttranslational mechanisms of aging". Biogerontology. 11 (4): 387–99. doi:10.1007/s10522-010-9262-y. PMID 20157779. S2CID 21455794.
- Strehler BL (1986). "Genetic instability as the primary cause of human aging". Experimental Gerontology. 21 (4–5): 283–319. doi:10.1016/0531-5565(86)90038-0. PMID 3545872. S2CID 34431271.
- Gavrilov LA, Gavrilova NA (2006). "Reliability Theory of Aging and Longevity". In Masoro EJ, Austad SN (eds.). Handbook of the Biology of Aging. San Diego, CA: Academic Press. pp. 3–42.
- Carroll B, Hewitt G, Korolchuk VI (2013). "Autophagy and ageing: implications for age-related neurodegenerative diseases". Essays in Biochemistry. 55: 119–31. doi:10.1042/bse0550119. PMID 24070476. S2CID 1603760.
- Cui M, Yu H, Wang J, Gao J, Li J (2013). "Chronic caloric restriction and exercise improve metabolic conditions of dietary-induced obese mice in autophagy correlated manner without involving AMPK". Journal of Diabetes Research. 2013: 852754. doi:10.1155/2013/852754. PMC 3671310. PMID 23762877.
- Lee JH, Kim EW, Croteau DL, Bohr VA (September 2020). "Heterochromatin: an epigenetic point of view in aging". Experimental & Molecular Medicine. 52 (9): 1466–1474. doi:10.1038/s12276-020-00497-4. PMID 32887933.
- Villeponteau B (1 July 1997). "The heterochromatin loss model of aging". Experimental Gerontology. Proceedings of the Third International Symposium on the Neurobiology and Neuroendocrinology of Aging. 32 (4–5): 383–94. doi:10.1016/S0531-5565(96)00155-6. PMID 9315443. S2CID 29375335.
- Tsurumi A, Li WX (July 2012). "Global heterochromatin loss: a unifying theory of aging?". Epigenetics. 7 (7): 680–8. doi:10.4161/epi.20540. PMC 3414389. PMID 22647267.
- Sturm Á, Ivics Z, Vellai T (May 2015). "The mechanism of ageing: primary role of transposable elements in genome disintegration". Cellular and Molecular Life Sciences. 72 (10): 1839–47. doi:10.1007/s00018-015-1896-0. PMID 25837999. S2CID 13241098.
- Elsner D, Meusemann K, Korb J (May 2018). "Longevity and transposon defense, the case of termite reproductives". Proceedings of the National Academy of Sciences of the United States of America. 115 (21): 5504–5509. doi:10.1073/pnas.1804046115. PMC 6003524. PMID 29735660.
- Sturm Á, Perczel A, Ivics Z, Vellai T (October 2017). "The Piwi-piRNA pathway: road to immortality". Aging Cell. 16 (5): 906–911. doi:10.1111/acel.12630. PMC 5595689. PMID 28653810.
- Bjorksten J, Tenhu H (1990). "The crosslinking theory of aging--added evidence". Experimental Gerontology. 25 (2): 91–5. doi:10.1016/0531-5565(90)90039-5. PMID 2115005. S2CID 19115146.
- Trifunovic A, Larsson NG (February 2008). "Mitochondrial dysfunction as a cause of ageing". Journal of Internal Medicine. 263 (2): 167–78. doi:10.1111/j.1365-2796.2007.01905.x. PMID 18226094. S2CID 28396237.
- Harman D (November 1981). "The aging process". Proceedings of the National Academy of Sciences of the United States of America. 78 (11): 7124–8. Bibcode:1981PNAS...78.7124H. doi:10.1073/pnas.78.11.7124. PMC 349208. PMID 6947277.
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M (October 2007). "Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress". Cell Metabolism. 6 (4): 280–93. doi:10.1016/j.cmet.2007.08.011. PMID 17908557.
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, et al. (August 2001). "Does oxidative damage to DNA increase with age?". Proceedings of the National Academy of Sciences of the United States of America. 98 (18): 10469–74. Bibcode:2001PNAS...9810469H. doi:10.1073/pnas.171202698. PMC 56984. PMID 11517304.
- Wolf FI, Fasanella S, Tedesco B, Cavallini G, Donati A, Bergamini E, Cittadini A (March 2005). "Peripheral lymphocyte 8-OHdG levels correlate with age-associated increase of tissue oxidative DNA damage in Sprague-Dawley rats. Protective effects of caloric restriction". Experimental Gerontology. 40 (3): 181–8. doi:10.1016/j.exger.2004.11.002. PMID 15763395. S2CID 23752647.
- Anson RM, Bohr VA (October 2000). "Mitochondria, oxidative DNA damage, and aging". Journal of the American Aging Association. 23 (4): 199–218. doi:10.1007/s11357-000-0020-y. PMC 3455271. PMID 23604866.
- Guarente L, Picard F (February 2005). "Calorie restriction--the SIR2 connection". Cell. 120 (4): 473–82. doi:10.1016/j.cell.2005.01.029. PMID 15734680. S2CID 14245512.
- Agarwal B, Baur JA (January 2011). "Resveratrol and life extension". Annals of the New York Academy of Sciences. 1215 (1): 138–43. Bibcode:2011NYASA1215..138A. doi:10.1111/j.1749-6632.2010.05850.x. PMID 21261652. S2CID 41701458.
- Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, et al. (June 2008). "Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function". Obesity. 16 (6): 1355–62. doi:10.1038/oby.2008.201. PMC 2748341. PMID 18421281.
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. (April 2006). "Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial". JAMA. 295 (13): 1539–48. doi:10.1001/jama.295.13.1539. PMC 2692623. PMID 16595757.
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. (July 2009). "Caloric restriction delays disease onset and mortality in rhesus monkeys". Science. 325 (5937): 201–4. Bibcode:2009Sci...325..201C. doi:10.1126/science.1173635. PMC 2812811. PMID 19590001.
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. (September 2012). "Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study". Nature. 489 (7415): 318–21. Bibcode:2012Natur.489..318M. doi:10.1038/nature11432. PMC 3832985. PMID 22932268.
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM (April 2014). "Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys". Nature Communications. 5: 3557. Bibcode:2014NatCo...5.3557C. doi:10.1038/ncomms4557. PMC 3988801. PMID 24691430.
- "There may be little advantage of moderate CR over modest CR—this would be an extremely important discovery and one that merits further investigation."
- Hayflick L (1994). How and why we age. New York: Ballantine Books. p. 261. ISBN 978-0-345-33918-8. OCLC 29908633.
- Bowman K, Delgado J, Henley WE, Masoli JA, Kos K, Brayne C, et al. (February 2017). "Obesity in Older People With and Without Conditions Associated With Weight Loss: Follow-up of 955,000 Primary Care Patients". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 72 (2): 203–209. doi:10.1093/gerona/glw147. PMC 5233914. PMID 27492450.
- Nakagawa S, Lagisz M, Hector KL, Spencer HG (June 2012). "Comparative and meta-analytic insights into life extension via dietary restriction". Aging Cell. 11 (3): 401–9. doi:10.1111/j.1474-9726.2012.00798.x. PMID 22268691. S2CID 19043668.
- Simpson SJ, Raubenheimer D (October 2009). "Macronutrient balance and lifespan". Aging. 1 (10): 875–80. doi:10.18632/aging.100098. PMC 2815731. PMID 20157561.
- Melnik BC (March 2012). "Leucine signaling in the pathogenesis of type 2 diabetes and obesity". World Journal of Diabetes. 3 (3): 38–53. doi:10.4239/wjd.v3.i3.38. PMC 3310004. PMID 22442749.
- Yan L, Lamb RF (August 2012). "Amino acid sensing and regulation of mTORC1". Seminars in Cell & Developmental Biology. 23 (6): 621–5. doi:10.1016/j.semcdb.2012.02.001. PMID 22342805.
- Rees K, Takeda A, Martin N, Ellis L, Wijesekara D, Vepa A, et al. (Cochrane Heart Group) (March 2019). "Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease". The Cochrane Database of Systematic Reviews. 3: CD009825. doi:10.1002/14651858.CD009825.pub3. PMC 6414510. PMID 30864165.
- Sofi F, Cesari F, Abbate R, Gensini GF, Casini A (September 2008). "Adherence to Mediterranean diet and health status: meta-analysis". BMJ. 337 (sep11 2): a1344. doi:10.1136/bmj.a1344. PMC 2533524. PMID 18786971.
- de Gaetano, Giovanni (29 August 2016). "Mediterranean diet associated with lower risk of early death in cardiovascular disease patients. European Society of Cardiology". ScienceDaily.
- Rowland R (15 February 2002). "Experts challenge study linking sleep, life span". CNN. Retrieved 29 October 2013.
- Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. (May 2004). "A prospective study of sleep duration and mortality risk in women". Sleep. 27 (3): 440–4. doi:10.1093/sleep/27.3.440. PMID 15164896.
- Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, Marmot MG (December 2007). "A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort". Sleep. 30 (12): 1659–66. doi:10.1093/sleep/30.12.1659. PMC 2276139. PMID 18246975. Lay summary – University of Warwick.
- Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB (July 2006). "Correlates of long sleep duration". Sleep. 29 (7): 881–9. doi:10.1093/sleep/29.7.881. PMC 3500381. PMID 16895254.; cf. Irwin MR, Ziegler M (February 2005). "Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics". Hypertension. 45 (2): 252–7. doi:10.1161/01.HYP.0000153517.44295.07. PMID 15642774.
- Yetish G, Kaplan H, Gurven M, Wood B, Pontzer H, Manger PR, et al. (November 2015). "Natural sleep and its seasonal variations in three pre-industrial societies". Current Biology. 25 (21): 2862–2868. doi:10.1016/j.cub.2015.09.046. PMC 4720388. PMID 26480842.
- Gremeaux V, Gayda M, Lepers R, Sosner P, Juneau M, Nigam A (December 2012). "Exercise and longevity". Maturitas. 73 (4): 312–7. doi:10.1016/j.maturitas.2012.09.012. PMID 23063021.
- Department of Health And Human Services, United States (1996). Physical Activity and Health. United States Department of Health. ISBN 978-1-4289-2794-0.
- Woods JA, Wilund KR, Martin SA, Kistler BM (February 2012). "Exercise, inflammation and aging". Aging and Disease. 3 (1): 130–40. PMC 3320801. PMID 22500274.
- Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. (August 2016). "Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013". BMJ. 354: i3857. doi:10.1136/bmj.i3857. PMC 4979358. PMID 27510511.
- Kramer, Arthur F.; Erickson, Kirk I. (1 August 2007). "Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function". Trends in Cognitive Sciences. 11 (8): 342–348. doi:10.1016/j.tics.2007.06.009. ISSN 1364-6613. PMID 17629545. S2CID 14669210.
- Notterman DA, Mitchell C (October 2015). "Epigenetics and Understanding the Impact of Social Determinants of Health". Pediatric Clinics of North America (Review). 62 (5): 1227–40. doi:10.1016/j.pcl.2015.05.012. PMC 4555996. PMID 26318949.
- Quinlan J, Tu MT, Langlois EV, Kapoor M, Ziegler D, Fahmi H, Zunzunegui MV (April 2014). "Protocol for a systematic review of the association between chronic stress during the life course and telomere length". Systematic Reviews (Review). 3 (40): 40. doi:10.1186/2046-4053-3-40. PMC 4022427. PMID 24886862.
- Noordam R, Gunn DA, Tomlin CC, Rozing MP, Maier AB, Slagboom PE, et al. (October 2012). "Cortisol serum levels in familial longevity and perceived age: the Leiden longevity study". Psychoneuroendocrinology. 37 (10): 1669–75. doi:10.1016/j.psyneuen.2012.02.013. PMID 22429748. S2CID 16189194.
- Lazzarino AI, Hamer M, Gaze D, Collinson P, Steptoe A (October 2013). "The association between cortisol response to mental stress and high-sensitivity cardiac troponin T plasma concentration in healthy adults". Journal of the American College of Cardiology. 62 (18): 1694–1701. doi:10.1016/j.jacc.2013.05.070. PMC 3807660. PMID 23810896.
- Holt-Lunstad J, Smith TB, Layton JB (July 2010). "Social relationships and mortality risk: a meta-analytic review". PLOS Medicine. 7 (7): e1000316. doi:10.1371/journal.pmed.1000316. PMC 2910600. PMID 20668659.
- "Social ties are good for your health". stanford.edu. Archived from the original on 11 September 2016.
- Koenig HG, King DE, Carson VB (2012). Handbook of religion and health (2nd ed.). New York: Oxford University Press. p. 476.
- "Married Vs Single: What Science Says Is Better For Your Health". medicaldaily.com. 2 April 2015.
- Shor E, Roelfs DJ, Bugyi P, Schwartz JE (July 2012). "Meta-analysis of marital dissolution and mortality: reevaluating the intersection of gender and age". Social Science & Medicine. 75 (1): 46–59. doi:10.1016/j.socscimed.2012.03.010. PMC 3881174. PMID 22534377.
- Lam YY, Peterson CM, Ravussin E (October 2013). "Resveratrol vs. calorie restriction: data from rodents to humans". Experimental Gerontology. 48 (10): 1018–24. doi:10.1016/j.exger.2013.04.005. PMID 23624181. S2CID 5392374.
- Pryor R, Cabreiro F (November 2015). "Repurposing metformin: an old drug with new tricks in its binding pockets". The Biochemical Journal. 471 (3): 307–22. doi:10.1042/bj20150497. PMC 4613459. PMID 26475449.
- Powers RW, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S (January 2006). "Extension of chronological life span in yeast by decreased TOR pathway signaling". Genes & Development. 20 (2): 174–84. doi:10.1101/gad.1381406. PMC 1356109. PMID 16418483.
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. (July 2009). "Rapamycin fed late in life extends lifespan in genetically heterogeneous mice". Nature. 460 (7253): 392–5. Bibcode:2009Natur.460..392H. doi:10.1038/nature08221. PMC 2786175. PMID 19587680. Lay summary – The Times (8 July 2009).
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. (February 2011). "Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 66 (2): 191–201. doi:10.1093/gerona/glq178. PMC 3021372. PMID 20974732.
- Ingram DK, Roth GS (March 2011). "Glycolytic inhibition as a strategy for developing calorie restriction mimetics". Experimental Gerontology (review). 46 (2–3): 148–54. doi:10.1016/j.exger.2010.12.001. PMID 21167272. S2CID 5634847.
- Tardif S, Ross C, Bergman P, Fernandez E, Javors M, Salmon A, et al. (May 2015). "Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 70 (5): 577–87. doi:10.1093/gerona/glu101. PMC 4400395. PMID 25038772.
- Callaway E (2010). "Telomerase reverses ageing process". Nature. doi:10.1038/news.2010.635.
- Blagosklonny MV (March 2009). "Validation of anti-aging drugs by treating age-related diseases". Aging. 1 (3): 281–8. doi:10.18632/aging.100034. PMC 2806014. PMID 20157517.
- Kogan V, Molodtsov I, Menshikov LI, Shmookler Reis RJ, Fedichev P (August 2015). "Stability analysis of a model gene network links aging, stress resistance, and negligible senescence". Scientific Reports. 5: 13589. arXiv:1408.0463. Bibcode:2015NatSR...513589K. doi:10.1038/srep13589. PMC 4551969. PMID 26316217.
- "Scientists' Open Letter on Aging" Archived 29 April 2015 at the Wayback Machine. imminst.org.
- Cornoni-Huntley J, Ostfeld AM, Taylor JO, Wallace RB, Blazer D, Berkman LF, et al. (February 1993). "Established populations for epidemiologic studies of the elderly: study design and methodology". Aging. 5 (1): 27–37. doi:10.1007/bf03324123. PMID 8481423. S2CID 26861993.
- Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, et al. (August 2007). "An Aging Interventions Testing Program: study design and interim report". Aging Cell. 6 (4): 565–75. doi:10.1111/j.1474-9726.2007.00311.x. hdl:2027.42/74625. PMID 17578509. S2CID 2409462.
- "Ageing". Gero. Retrieved 4 February 2015.
- "Science for Life Extension". Science against aging foundation. Archived from the original on 18 February 2015. Retrieved 3 February 2015.
- "FAQ". Palo Alto Longevity Prize. Retrieved 1 October 2014.
- Vance A (9 September 2014). "Silicon Valley Investor Backs $1 Million Prize to End Death". Bloomberg Businessweek. Retrieved 1 October 2014.
- "$1 Million Longevity Prize Seeks To "Hack The Aging Code"" (Press release). Yahoo! Finance. 9 September 2014. Archived from the original on 6 October 2014. Retrieved 1 October 2014.CS1 maint: bot: original URL status unknown (link)
- Kinney A (14 September 2014). "Silicon Valley launches another bid to 'hack' aging, cheat death". San Jose Mercury News. Retrieved 1 October 2014.
- Thorp V (23 November 2014). "The Palo Alto Prize: A 'Moonshot' at Increasing Longevity". Palo Alto Pulse. Retrieved 8 December 2014.
- Phillips, Judith, Kristine Ajrouch, and Sarah Hillcoat-Nallétamby (2010) Key Concepts in Social Gerontology. SAGE Publications. ISBN 978-1-4462-0428-3. pp. 12–13.
- Levy BR, Slade MD, Chang ES, Kannoth S, Wang SY (January 2020). "Ageism Amplifies Cost and Prevalence of Health Conditions". The Gerontologist. 60 (1): 174–181. doi:10.1093/geront/gny131. PMC 7182003. PMID 30423119.
- "Ageing in the Twenty-First Century". UNFPA. 2012.
- LO W (2015). "The music culture of older adults in Cantonese operatic singing lessons". Ageing and Society. 35 (8): 1614–34. doi:10.1017/S0144686X14000439. S2CID 144367063.
- Vincent JA (December 2005). "Understanding generations: political economy and culture in an ageing society". The British Journal of Sociology. 56 (4): 579–99. doi:10.1111/j.1468-4446.2005.00084.x. PMID 16309437. S2CID 1775770.
- "Population Ageing and Development". UNFPA. 2002.
- "Ageing". unfpa.org. UNFPA – United Nations Population Fund.
- "UN Human Development Report 2005" (PDF). United Nations Development Programme. Archived from the original (PDF) on 27 May 2008. Retrieved 7 October 2010.
- Chosewood, L. Casey. "Safer and Healthier at Any Age: Strategies for an Aging Workforce". NIOSH Science Blog. National Institute for Occupational Safety and Health. Retrieved 6 August 2012.
- Basakha M, Yavari K, Sadeghi H, Naseri A (2015). "Population Aging And Iran's Non-Oil Economic Growth". Payavard Salamat. 9 (2): 131–46.
- Basakha M, Yavari K, Sadeghi H, Naseri A (2014). "Health care cost disease as a threat to Iranian aging society". Journal of Research in Health Sciences. 14 (2): 152–6. PMID 24728752.
- Scheid TL, Brown TN (2010). A Handbook for the Study of Mental Health (Second ed.). New York: Cambridge University Press.
- Panek PE, Hayslip B (1989). Adult development and aging. San Francisco: Harper & Row. ISBN 978-0-06-045012-0.
- Wejbrandt A (December 2014). "Defining aging in cyborgs: a bio-techno-social definition of aging". Journal of Aging Studies. 31: 104–9. doi:10.1016/j.jaging.2014.09.003. PMID 25456627.
- Emmanuel EJ (October 2014). "Why I hope to die at 75: An argument that society and families – and you – will be better off if nature takes its course swiftly and promptly". The Atlantic. Retrieved 7 April 2015.
- Faria MA (2015). "Bioethics and why I hope to live beyond age 75 attaining wisdom!: A rebuttal to Dr. Ezekiel Emanuel's 75 age limit". Surgical Neurology International. 6: 35. doi:10.4103/2152-7806.152733. PMC 4360549. PMID 25789197.
- Faria MA (2015). "Longevity and compression of morbidity from a neuroscience perspective: Do we have a duty to die by a certain age?". Surgical Neurology International. 6: 49. doi:10.4103/2152-7806.154273. PMC 4392568. PMID 25883841.
- Saltman RB, Dubois HF, Chawla M (2006). "The impact of aging on long-term care in Europe and some potential policy responses". International Journal of Health Services. 36 (4): 719–46. doi:10.2190/AUL1-4LAM-4VNB-3YH0. PMID 17175843. S2CID 45396303.
- Reinhardt UE (2003). "Does the aging of the population really drive the demand for health care?". Health Affairs. 22 (6): 27–39. doi:10.1377/hlthaff.22.6.27. PMID 14649430.
- Meara E, White C, Cutler DM (2004). "Trends in medical spending by age, 1963-2000". Health Affairs. 23 (4): 176–83. doi:10.1377/hlthaff.23.4.176. PMID 15318578.
- Kattimani V, Tiwari RV, Gufran K, Wasan B, Shilpa PH, Khader AA (March 2019). "Botulinum Toxin Application in Facial Esthetics and Recent Treatment Indications (2013-2018)". Journal of International Society of Preventive & Community Dentistry. 9 (2): 99–105. doi:10.4103/jispcd.JISPCD_430_18. PMC 6489509. PMID 31058058.
Standards of beauty have changed through centuries with increased awareness about esthetics.
- Juhász ML, Levin MK, Marmur ES (June 2018). "The use of natural ingredients in innovative Korean cosmeceuticals". Journal of Cosmetic Dermatology. 17 (3): 305–312. doi:10.1111/jocd.12492. PMID 29363245. S2CID 25982162.
- Juhász ML, Levin MK, Marmur ES (June 2018). "The use of natural ingredients in innovative Korean cosmeceuticals". Journal of Cosmetic Dermatology. 17 (3): 305–312. doi:10.1111/jocd.12492. PMID 29363245. S2CID 25982162.
- Sabatini S, Silarova B, Martyr A, Collins R, Ballard C, Anstey KJ, et al. (August 2020). "Associations of Awareness of Age-Related Change With Emotional and Physical Well-being: A Systematic Review and Meta-analysis". The Gerontologist. 60 (6): e477–e490. doi:10.1093/geront/gnz101. PMC 7427487. PMID 31350849.
- Idler EL (2003). "Discussion: Gender Differences in Self-Rated Health, in Mortality, and in the Relationship Between the Two". The Gerontologist. 43 (3): 372–75. doi:10.1093/geront/43.3.372.
- Deeg DJ, Bath PA (June 2003). "Self-rated health, gender, and mortality in older persons: introduction to a special section". The Gerontologist. 43 (3): 369–71. doi:10.1093/geront/43.3.369. PMID 12810900.
- Benyamini Y, Blumstein T, Lusky A, Modan B (June 2003). "Gender differences in the self-rated health-mortality association: is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival?". The Gerontologist. 43 (3): 396–405, discussion 372–5. doi:10.1093/geront/43.3.396. PMID 12810904.
- Kunzmann U, Little TD, Smith J (September 2000). "Is age-related stability of subjective well-being a paradox? Cross-sectional and longitudinal evidence from the Berlin Aging Study". Psychology and Aging. 15 (3): 511–26. doi:10.1037/0882-7974.15.3.511. PMID 11014714.
- Jylhä M, Guralnik JM, Balfour J, Fried LP (October 2001). "Walking difficulty, walking speed, and age as predictors of self-rated health: the women's health and aging study". The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 56 (10): M609-17. doi:10.1093/gerona/56.10.m609. PMID 11584033.
- Heckhausen J (1999). Developmental Regulation in Adulthood: Age-Normative and Sociostructural Constraints as Adaptive Challenges. Cambridge University Press. ISBN 978-0-521-02713-7.
- Sargent-Cox KA, Anstey KJ, Luszcz MA (September 2008). "Determinants of self-rated health items with different points of reference: implications for health measurement of older adults". Journal of Aging and Health. 20 (6): 739–61. doi:10.1177/0898264308321035. PMID 18625760. S2CID 34866893.
- Idler EL (November 1993). "Age differences in self-assessments of health: age changes, cohort differences, or survivorship?". Journal of Gerontology. 48 (6): S289-300. doi:10.1093/geronj/48.6.s289. PMID 8228003.
- Williamson JD, Fried LP (December 1996). "Characterization of older adults who attribute functional decrements to "old age"". Journal of the American Geriatrics Society. 44 (12): 1429–34. doi:10.1111/j.1532-5415.1996.tb04066.x. PMID 8951311. S2CID 21027678.
- Baltes PB, Baltes MM (1990). "Psychological perspectives on successful aging: The model of selective optimization with compensation". In Baltes PB, Baltes MM (eds.). Successful Aging. pp. 1–34. doi:10.1017/CBO9780511665684.003. ISBN 978-0-511-66568-4.
- Rowe JW, Kahn RL (July 1987). "Human aging: usual and successful". Science. 237 (4811): 143–9. Bibcode:1987Sci...237..143R. doi:10.1126/science.3299702. PMID 3299702.
- Job 14:5–7 A man’s days are numbered. You know the number of his months. He cannot live longer than the time You have set. So now look away from him that he may rest, until he has lived the time set for him like a man paid to work. For there is hope for a tree, when it is cut down, that it will grow again, and that its branches will not stop growing
External links
- Global AgeWatch Statistics on population ageing and life expectancy
- HelpAge International and UNFPA (2012). Ageing in the 21st Century – A Celebration and a Challenge.