RTI-121
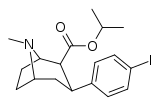
(–)-2β-Carboisopropoxy-3β-(4-iodophenyl)tropane (RTI-4229-121, IPCIT) is a stimulant drug used in scientific research, which was developed in the early 1990s.[1] RTI-121 is a phenyltropane based, highly selective dopamine reuptake inhibitor[2] and is derived from methylecgonidine. RTI-121 is a potent and long-lasting stimulant, producing stimulant effects for more than 10 hours after a single dose in mice[3] which would limit its potential uses in humans, as it might have significant abuse potential if used outside a medical setting. However RTI-121 occupies the dopamine transporter more slowly than cocaine, and so might have lower abuse potential than cocaine itself.[4]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H24INO2 |
| Molar mass | 413.299 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Uses
RTI-121 is mainly used in scientific research into the dopamine reuptake transporter. It is more selective for the dopamine transporter than other DAT radioligands such as β-CIT, and so has less nonspecific binding and produces "cleaner" images.[5][6] Various radiolabelled forms of RTI-121 (with different radioactive isotopes of iodine used depending on the application) are used in both humans and animals to map the distribution of dopamine transporters in the brain.[7][8]
Legal status
RTI-121 not specified as controlled substance in any country as of 2007. Some jurisdictions such as the United States, Australia, and New Zealand, however, might however consider RTI-121 to be a controlled substance analogue of cocaine on the grounds of its related chemical structure.
See also
- RTI-55
- List of cocaine analogues
- List of Phenyltropanes
References
- Scheffel U, Dannals RF, Wong DF, Yokoi F, Carroll FI, Kuhar MJ (November 1992). "Dopamine transporter imaging with novel, selective cocaine analogs". NeuroReport. 3 (11): 969–72. doi:10.1097/00001756-199211000-00005. PMID 1482766.
- Boja JW, Cadet JL, Kopajtic TA, Lever J, Seltzman HH, Wyrick CD, et al. (April 1995). "Selective labeling of the dopamine transporter by the high affinity ligand 3 beta-(4-[125I]iodophenyl)tropane-2 beta-carboxylic acid isopropyl ester". Molecular Pharmacology. 47 (4): 779–86. PMID 7723739.
- Fleckenstein AE, Kopajtic TA, Boja JW, Carroll FI, Kuhar MJ (September 1996). "Highly potent cocaine analogs cause long-lasting increases in locomotor activity". European Journal of Pharmacology. 311 (2–3): 109–14. doi:10.1016/0014-2999(96)00423-2. PMID 8891589.
- Stathis M, Scheffel U, Lever SZ, Boja JW, Carroll FI, Kuhar MJ (June 1995). "Rate of binding of various inhibitors at the dopamine transporter in vivo". Psychopharmacology. 119 (4): 376–84. doi:10.1007/BF02245852. PMID 7480516. S2CID 20022021.
- Scanley BE, al-Tikriti MS, Gandelman MS, Laruelle M, Zea-Ponce Y, Baldwin RM, et al. (January 1995). "Comparison of [123I]beta-CIT and [123I]IPCIT as single-photon emission tomography radiotracers for the dopamine transporter in nonhuman primates". European Journal of Nuclear Medicine. 22 (1): 4–11. doi:10.1007/BF00997241. PMID 7698153. S2CID 20406294.
- Scanley BE, Gandelman MS, Laruelle M, Al-Tikriti MS, Baldwin RM, Zoghbi SS, et al. (January 2000). "[123I]IPCIT and [123I]beta-CIT as SPECT tracers for the dopamine transporter: a comparative analysis in nonhuman primates". Nuclear Medicine and Biology. 27 (1): 13–21. doi:10.1016/s0969-8051(99)00083-9. PMID 10755641.
- Chen NH, Ding JH, Wang YL (March 1997). "Characterization of [125I]RTI-121 binding to dopamine transporter in vitro". Zhongguo Yao Li Xue Bao = Acta Pharmacologica Sinica. 18 (2): 115–20. PMID 10072960.
- Lever JR, Scheffel U, Stathis M, Seltzman HH, Wyrick CD, Abraham P, et al. (April 1996). "Synthesis and in vivo studies of a selective ligand for the dopamine transporter: 3 beta-(4-[125I]iodophenyl) tropan-2 beta-carboxylic acid isopropyl ester ([125I]RTI-121)". Nuclear Medicine and Biology. 23 (3): 277–84. doi:10.1016/0969-8051(95)02074-8. PMID 8782237.