Epibatidine
Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus.[1] It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine. [2]
-Epibatidine_Structural_Formulae_V.1.svg.png.webp) | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H13ClN2 |
| Molar mass | 208.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Epibatidine is toxic. Its toxicity stems from its ability to interact with nicotinic and muscarinic acetylcholine receptors. These receptors are involved in the transmission of painful sensations, and in movement, among other functions. Epibatidine then causes numbness, and, eventually, paralysis. Doses are lethal when the paralysis causes respiratory arrest. Originally, it was thought that epibatidine could be useful as a drug. However, because of its unacceptable therapeutic index, it is no longer being researched for potential therapeutic uses.[3]
History
Epibatidine was discovered by John W. Daly in 1974. It was isolated from the skin of Epipdobates anthonyi frogs collected by Daly and colleague, Charles Myers. Between 1974 and 1979, Daly and Myers collected the skins of nearly 3000 frogs from various sites in Ecuador, after finding that a small injection of a preparation from their skin caused analgesic (painkilling) effects in mice that resembled those of an opioid.[2] Despite its common name - Anthony's Poison Arrow frog - suggesting that it was used by natives when hunting,[4] a paper written by Daly in 2000 claimed that there was no local folklore or folk medicine surrounding the frogs and that they were considered largely unimportant by the locals.[5]

The structure of epibatidine was elucidated in 1992, an effort hindered by E. anthonyi gaining IUCN protected status in 1984.[5] Furthermore, these frogs do not produce the toxin when bred and reared in captivity, because they do not synthesize epibatidine themselves. Like other poison dart frogs, they instead obtain it through their diet and then sequester it on their skin. Likely dietary sources are beetles, ants, mites, and flies.[6] Overcoming the difficulties, the structure was eventually determined, and the first synthesis of epibatidine was completed in 1993. Many other synthesis methods have been developed since.[5]
Because of its analgesic effect, there was intense interest in epibatidine's use as a drug, because it was found not to be an opioid.[2] This meant that it could potentially be used without fear of addiction. However, it was soon found that it cannot be used in humans because the dose resulting in toxic symptoms is too low for it to be safe.[7]
Synthesis
Several total synthesis routes have been devised due to the relative scarcity of epibatidine in nature.[8]
After the discovery of the structure of epibatidine, more than fifty ways to synthesize it in the laboratory have been devised. In the first reported example, a nine-step procedure produces the substance as a racemate (in contrast, the naturally occurring compound is the (+)-enantiomer; the (−)-enantiomer does not occur naturally). It was later determined that the (+) and (-) enantiomers had equivalent analgesic as well as toxic effects. The process has proven to be quite productive, with a yield of about 40%.[9][10][11]
An enantioselective synthesis reported by E J Corey starting from chloronicotinaldehyde is outlined below:
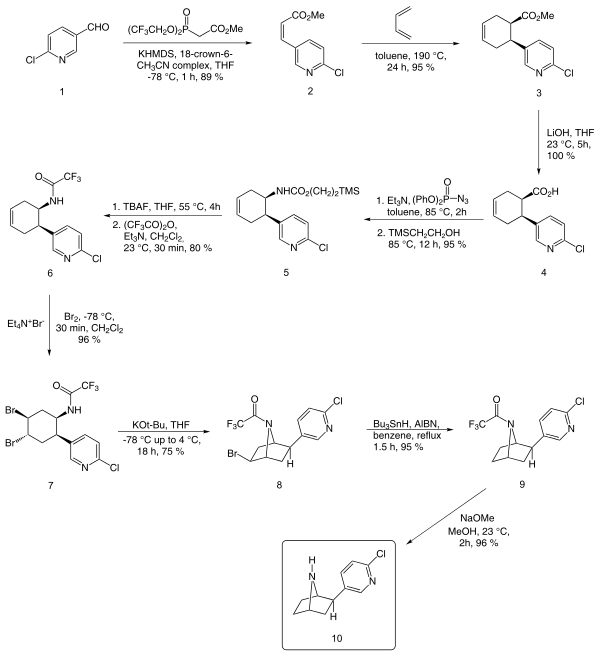
In addition to Corey's method, other notable methods include those of Broka,[12] Huang and Shen,[13] and Clayton and Regan.[10]
Synthetic analogs
A number of approaches to discovering structural analogs of epibatine that maintain analgesics effects, but without the toxicity, have been attempted.[14] For example, Abbott Laboratories has produced derivatives of epibatidine including ABT-594.[15] Other epibatidine analogs include ABT-418, epiboxidine and their derivatives.[14][16][17][18][19] A synthesis of epibatidine, utilizing a microbial hydroxylation of an unactivated carbon in a 7-azanorbornane was published in 1999.[20]
Chemical structure
Epibatidine is a pyridine with a structure similar to that of nicotine.[21] It is a hygroscopic oily substance which is a base.
Biological effects
Mechanism of action
Epibatidine has two mechanisms of action: it can bind to either nicotinic acetylcholine receptors (nAChR) or muscarinic acetylcholine receptors (mAChR). Specifically, the analgesic property of epibatidine is believed to take place by its binding to the α4/β2 subtype of nicotinic receptors. Epibatidine also binds to the α3/β4 subtype and to a much lesser extent α7 receptors (affinity 300-fold less than for α4/β2)[22] The rank order of affinities is αε > αγ > αδ.[23]
Nicotinic acetylcholine receptors are found in the post-synaptic membranes of nerve cells. They propagate neurotransmission in the central and peripheral nervous system. When neurotransmitters bind to these receptors, ion channels open, allowing Na+ and Ca2+ ions to move across the membrane. This depolarizes the post-synaptic membrane, inducing an action potential that propagates the signal. This signal will ultimately induce release of dopamine and norepinephrine, resulting in an antinociceptive effect on the organism. The usual neurotransmitter for nAChR is acetylcholine. However, other substances (such as epibatidine and nicotine) are also able to bind to the receptor and induce a similar, if not identical, response. Epibatidine has an extremely high affinity for nAChRs, depending on the receptor subtype, from 0.05 nM at the α4β2 subtype to 22 nM at the α7 subtype. Affinity as well as efficacy (and thus also potency) are much higher than for nicotine.[9]
The paralytic property of epibatidine takes place after its binding to muscle-type nicotinic receptors.
Low doses of epibatidine will only affect the nAChRs, due to a higher affinity to nAChRs than to mAChRs. Higher doses, however, will cause epibatidine to bind to the mAChRs.
Both (+)- and (-)-enantiomers of epibatidine are biologically active, and both have similar binding affinities to nAChRs[9] Only the (+)-enantiomer does not induce tolerance. While this may be a potential therapeutic advantage over morphine, epibatidine has not entered clinical trials because even very small doses are lethal to rodents.[24]
Symptoms
Epibatidine has several toxic consequences. Empirically proven effects include splanchnic sympathetic nerve discharge and increased arterial pressure.[21] The nerve discharge effects can cause antinociception partially mediated by agonism of central nicotinic acetylcholine receptors at low doses of epibatidine; 5 µg/kg.[25] At higher doses, however, epibatidine will cause paralysis and loss of consciousness, coma and eventually death. The median lethal dose (LD50) of epibatidine lies between 1.46 µg/kg and 13.98 µg/kg.[26] This makes epibatidine somewhat more toxic than dioxin (with an average LD50 of 22.8 µg/kg). Due to the small difference between its toxic concentration and antinociceptive concentration, its therapeutic uses are very limited.
In research on mice, administration of doses greater than 5 μg/kg of epibatidine caused a dose-dependent paralyzing effect on the organism. With doses over 5 μg/kg, symptoms included hypertension (increased blood pressure), paralysis in the respiratory system, seizures, and, ultimately, death. The symptoms do, however, change drastically when lower doses are given. Mice became resistant to pain and heat with none of the negative effects of higher doses.
Pharmacology
Epibatidine most effectively enters the body through injection.[27] In vitro studies seem to suggest that epibatidine is hardly, if at all, metabolized in the human body.[28]
Also there is currently little information on the path of clearance from the body. Maximum concentration in the brain is reached at about 30 minutes after entering the body.[9]
Potential medical uses
Epibatidine has a high analgesic potency, as stated above. Studies show it has a potency at least 200 times that of morphine.[9] As the compound was not addictive nor did it cause habituation,, it was initially thought to be very promising to replace morphine as a painkiller. However, the therapeutic concentration is very close to the toxic concentration. This means that even at a therapeutic dose (5 µg/kg[25]), some epibatidine might bind to the muscarinic acetylcholine receptors and cause adverse effects, such as hypertension, bradycardia and muscular paresis.[21]
Compared to the gold standard in pain management, morphine, epibatidine needed only 2.5 μg/kg to initiate a pain-relieving effect whilst the same effect required approximately 10 mg/kg of morphine (4,000 times the efficacy.) Currently, only rudimentary research into epibatidine's effects has yet been performed; the drug has been administered only to rodents for analysis at this time.[11]
Antidote
The antidote to epibatidine is mecamylamine,[29] a nicotinic acetylcholine receptor antagonist that is non-selective and non-competitive.[30]
See also
- 6-Chloronicotine
- Arrow poison
- Batrachotoxin (and closely related Homobatrachotoxin)
- Tetrodotoxin
- Phantasmidine
References
- Fitch, R. W.; Spande, T. F.; Garraffo, H. M.; Yeh, H. J. C.; Daly, J. W. (2010). "Phantasmidine: An Epibatidine Congener from the Ecuadorian Poison Frog Epipedobates anthonyi⊥". Journal of Natural Products. 73 (3): 331–7. doi:10.1021/np900727e. PMC 2866194. PMID 20337496.
- "Epibatidine: From Frog Alkaloid to Analgesic Clinical Candidates. A Testimonial to "True Grit"!" (PDF). Heterocycles. pp. 207–217. Retrieved 2015-05-06.
- Schwarcz, Joe (2012). The Right Chemistry. Random House.
- "Epipedobates anthonyi". Retrieved 2015-05-06.
- Daly & Garraffo (2000). "Alkaloids from frog skin: the discovery of epibatidine and the potential for developing novel non-opioid analgesics". Natural Product Reports. 17 (2): 131–135. doi:10.1039/a900728h. PMID 10821107.
- Elizabeth Norton Lasley (December 1999). "Having Their Toxins and Eating Them Too Study of the natural sources of many animals' chemical defenses is providing new insights into nature's medicine chest". BioScience. Oxford Journals. 45 (12): 945–950. doi:10.1525/bisi.1999.49.12.945. Retrieved 2015-05-06.
- Diana L. Donnelly-Roberts; Pamela S. Puttfarcken; Theresa A. Kuntzweiler; Clark A. Briggs; David J. Anderson; Jeffrey E. Campbell; Marietta Piattoni-Kaplan; David G. Mckenna; James T. Wasicak; Mark W. Holladay; Michael Williams & Stephen P. Arneric (May 1998). "ABT-594 [(R)-5-(2-Azetidinylmethoxy)-2-Chloropyridine]: A Novel, Orally Effective Analgesic Acting via Neuronal Nicotinic Acetylcholine Receptors: I. In Vitro Characterization". The Journal of Pharmacology and Experimental Therapeutics. 285 (2): 777–786. PMID 9580626.
- Olivo, Horacio F.; Hemenway, Michael S. (2002). "Recent syntheses of epibatidine. A review". Organic Preparations and Procedures International. 34 (1): 1–26. doi:10.1080/00304940209355744. S2CID 98696766.
- "Epibatidine and pain" (PDF). British Journal of Anaesthesia 1998. pp. 69–76. Retrieved 2014-03-12.
- Simon C. Clayton; Andrew C. Regan (1993). "A total synthesis of (±)-epibatidine". Tetrahedron Letters. 34 (46): 7493–7496. doi:10.1016/S0040-4039(00)60162-4.
- Broka, C.A. Synthetic Approaches to Epibatidine. Med. Chem. Res. 1994, 4, 449-460.
- Broka, C.A. (1993). "Total synthesis of epibatidine". Tetrahedron Lett. 34 (20): 3251–3254. doi:10.1016/s0040-4039(00)73674-4.
- Huang, D.F.; Shen, T.Y. (1993). "A versatile total synthesis of epibatidine and analogs". Tetrahedron Lett. 34 (28): 4477–4480. doi:10.1016/0040-4039(93)88063-o.
- Archived December 5, 2010, at the Wayback Machine
- "Deriving a non-opiate painkiller [ABT-594] from Epipedobates tricolor". Mongabay.com. Retrieved 2014-03-12.
- Rizzi, Luca; Dallanoce, Clelia; Matera, Carlo; Magrone, Pietro; Pucci, Luca; Gotti, Cecilia; Clementi, Francesco; De Amici, Marco (2008-08-15). "Epiboxidine and novel-related analogues: A convenient synthetic approach and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes" (PDF). Bioorganic & Medicinal Chemistry Letters. 18 (16): 4651–4654. doi:10.1016/j.bmcl.2008.07.016. hdl:2434/59291. PMID 18644719.
- Dallanoce, Clelia; Matera, Carlo; Amici, Marco De; Rizzi, Luca; Pucci, Luca; Gotti, Cecilia; Clementi, Francesco; Micheli, Carlo De (2012-07-01). "The enantiomers of epiboxidine and of two related analogs: Synthesis and estimation of their binding affinity at α4β2 and α7 neuronal nicotinic acetylcholine receptors". Chirality. 24 (7): 543–551. doi:10.1002/chir.22052. ISSN 1520-636X. PMID 22566097.
- Dallanoce, Clelia; Matera, Carlo; Pucci, Luca; Gotti, Cecilia; Clementi, Francesco; Amici, Marco De; Micheli, Carlo De (2012-01-15). "Synthesis and binding affinity at α4β2 and α7 nicotinic acetylcholine receptors of new analogs of epibatidine and epiboxidine containing the 7-azabicyclo[2.2.1]hept-2-ene ring system". Bioorganic & Medicinal Chemistry Letters. 22 (2): 829–832. doi:10.1016/j.bmcl.2011.12.052. PMID 22222032.
- Dallanoce, Clelia; Magrone, Pietro; Matera, Carlo; Lo Presti, Leonardo; De Amici, Marco; Riganti, Loredana; Clementi, Francesco; Gotti, Cecilia; De Micheli, Carlo (2010-12-01). "Synthesis of novel chiral Δ2-isoxazoline derivatives related to ABT-418 and estimation of their affinity at neuronal nicotinic acetylcholine receptor subtypes". European Journal of Medicinal Chemistry. 45 (12): 5594–5601. doi:10.1016/j.ejmech.2010.09.009. PMID 20932609.
- Olivo, Horacio F.; Hemenway, Michael S. (1999-11-01). "Total Synthesis of (±)-Epibatidine Using a Biocatalytic Approach". The Journal of Organic Chemistry. 64 (24): 8968–8969. doi:10.1021/jo991141q. ISSN 0022-3263. PMID 11674810.
- Fisher M, Huangfu D, Shen TY, Guyenet PG (1994). "Epibatidine, an alkaloid from the poison frog Epipedobates tricolor, is a powerful ganglionic depolarizing agent". J Pharmacol Exp Ther. 270 (2): 702–7. PMID 8071862.
- Traynor, John R. (1998). "Epibatidine and pain". British Journal of Anaesthesia. 81 (1): 69–76. doi:10.1093/bja/81.1.69. PMID 9771274.
- Prince, Richard J.; Sine, Steven M. (1998-04-03). "Epibatidine Binds with Unique Site and State Selectivity to Muscle Nicotinic Acetylcholine Receptors". Journal of Biological Chemistry. 273 (14): 7843–7849. doi:10.1074/jbc.273.14.7843. ISSN 0021-9258. PMID 9525877.
- Abby Olena. How Poison Frogs Avoid Poisoning Themselves. The Scientist, September 21, 2017H. http://www.the-scientist.com/?articles.view/articleNo/50409/title/How-Poison-Frogs-Avoid-Poisoning-Themselves/
- Badio B, Daly JW. Epibatidine, a potent analgetic and nicotinic agonist, Molecular Pharmacology 1994; 45: 563-569
- Sihver, Acta (2002). "Neurologica Scandinavica, Ligands for in vivo imaging on nicotinic receptor subtypes in Alzheimer brain". Interscience.wiley.com. Retrieved 2014-03-12.
- Sullivan, J.P.; Bannon, A.W. (1996). "Epibatidine: Pharmacological Properties of a Novel Nicotinic Acetylcholine Receptor Agonist and Analgesic Agent". CNS Drug Reviews. 2 (1): 21–39. doi:10.1111/j.1527-3458.1996.tb00288.x.
- Watt A. P., Hitzel L., Morrison D., Locker K. L., Determination of the in vitro metabolism of (1)- and (2)-epibatidine, Journal of Chromatography A; 896: 229–238, 2000.
- Damaj, M.I.; et al. (1994). "Pharmacological effects of epibatidine optical enantiomers". Brain Research. 664 (1): 34–40. doi:10.1016/0006-8993(94)91950-x. PMID 7895043. S2CID 46489298.
- Bacher, I.; et al. (November 2009). "Mecamylamine - a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders". Expert Opinion on Pharmacotherapy. 10 (16): 2709–2721. doi:10.1517/14656560903329102. PMID 19874251. S2CID 25690407.