Omiloxetine
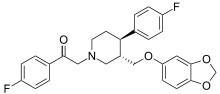
Omiloxetine (omiloextinum, omiloxetino INN)[1] was a selective serotonin reuptake inhibitor drug candidate that underwent preclinical development by the Spanish pharmaceutical company, Ferrer Internacional, until 2005, when it was abandoned.[2][3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H25F2NO4 |
| Molar mass | 465.497 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.3±0.1 g/cm3 |
| Melting point | 228.65 °C (443.57 °F) |
| Boiling point | 587.2 °C (1,089.0 °F) |
| Solubility in water | 0.0015 mg/mL (20 °C) |
| |
| |
References
- "Proposed INN: List 76" (PDF). WHO Drug Information. 10 (4): 212–213. 1996.
- Terencio J (2005-12-05). "Omiloxetine - Ferrer licensing offer, worldwide". R & D Focus Drug News. Archived from the original on 2016-03-07. Retrieved 2012-05-19.
- De Lecea C (2003-12-08). "Omiloxetine - Ferrer partnering opportunity, worldwide Ferrer preclinical data". R & D Focus Drug News. Archived from the original on 2016-03-07. Retrieved 2012-05-19.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.