Magnolol
Magnolol is an organic compound that is classified as lignan. It is a bioactive compound found in the bark of the Houpu magnolia (Magnolia officinalis) or in M. grandiflora.[2] The compound exists at the level of a few percent in the bark of species of magnolia, the extracts of which have been used in traditional Chinese and Japanese medicine. In addition to magnolol, related lignans occur in the extracts including honokiol, which is an isomer of magnolol.
 | |
| Names | |
|---|---|
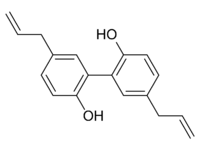
| IUPAC name
4-Allyl-2-(5-allyl-2-hydroxy-phenyl)phenol | |
| Other names
Dehydrodichavicol 5,5'-Diallyl-2,2'-dihydroxybiphenyl 5,5'-Diallyl-2,2'-biphenyldiol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.127.908 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H18O2 | |
| Molar mass | 266.340 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bioactivity
It is known to act on the GABAA receptors in rat cells in vitro[3] as well as having antifungal properties.[4] Magnolol has a number of osteoblast-stimulating and osteoclast-inhibiting activities in cell culture and has been suggested as a candidate for screening for anti-osteoporosis activity.[5] It has anti-periodontal disease activity in a rat model.[6] Structural analogues have been studied and found to be strong allosteric modulators of GABAA.[7] Magnolol is also binding in dimeric mode to PPARγ, acting as an agonist of this nuclear receptor.[8]
References
- Magnolol at Sigma-Aldrich
- Lee, Young-Jung; Lee, Yoot Mo; Lee, Chong-Kil; Jung, Jae Kyung; Han, Sang Bae; Hong, Jin Tae (2011). "Therapeutic applications of compounds in the Magnolia family". Pharmacology & Therapeutics. 130 (2): 157–76. doi:10.1016/j.pharmthera.2011.01.010. PMID 21277893.
- Ai, Jinglu; Wang, Xiaomei; Nielsen, Mogens (2001). "Honokiol and Magnolol Selectively Interact with GABAA Receptor Subtypes in vitro". Pharmacology. 63 (1): 34–41. doi:10.1159/000056110. PMID 11408830. S2CID 19327464.
- Bang, Kyu Ho; Kim, Yoon Kwan; Min, Byung Sun; Na, Min Kyun; Rhee, Young Ha; Lee, Jong Pill; Bae, Ki Hwan (2000). "Antifungal activity of magnolol and honokiol". Archives of Pharmacal Research. 23 (1): 46–9. doi:10.1007/BF02976465. PMID 10728656. S2CID 22754315.
- Kwak, Eun Jung; Lee, Young Soon; Choi, Eun Mi (2012). "Effect of Magnolol on the Function of Osteoblastic MC3T3-E1 Cells". Mediators of Inflammation. 2012: 1–7. doi:10.1155/2012/829650. PMC 3306956. PMID 22474400.
- Lu, Sheng-Hua; Huang, Ren-Yeong; Chou, Tz-Chong (2013). "Magnolol Ameliorates Ligature-Induced Periodontitis in Rats and Osteoclastogenesis: In Vivo and in Vitro Study". Evidence-Based Complementary and Alternative Medicine. 2013: 1–12. doi:10.1155/2013/634095. PMC 3618931. PMID 23573141.
- Fuchs, A; Baur, R; Schoeder, C; Sigel, E; Müller, CE (Dec 15, 2014). "Structural analogues of the natural products magnolol and honokiol as potent allosteric potentiators of GABAA receptors". Bioorg Med Chem. 22 (24): 6908–17. doi:10.1016/j.bmc.2014.10.027. PMID 25456080.
- Dreier D, Latkolik S, Rycek L, Schnürch M, Dymáková A, Atanasov AG, Ladurner A, Heiss EH, Stuppner H, Schuster D, Mihovilovic MD, Dirsch VM. Linked magnolol dimer as a selective PPARγ agonist - Structure-based rational design, synthesis, and bioactivity evaluation. Sci Rep. 2017 Oct 20;7(1):13002. doi: 10.1038/s41598-017-12628-5.
Further reading
- Squires, Richard F.; Ai, Jinglu; Witt, Michael-Robin; Kahnberg, Pia; Saederup, Else; Sterner, Olov; Nielsen, Mogens (1999). "Honokiol and magnolol increase the number of 3H muscimol binding sites three-fold in rat forebrain membranes in vitro using a filtration assay, by allosterically increasing the affinities of low-affinity sites". Neurochemical Research. 24 (12): 1593–602. doi:10.1023/A:1021116502548. PMID 10591411. S2CID 9070185.
- Rycek L, Puthenkalam R, Schnürch M, Ernst M, Mihovilovic MD (2015). "Metal-assisted synthesis of unsymmetrical magnolol and honokiol analogs and their biological assessment as GABAA receptor ligands". Bioorg. Med. Chem. Lett. 25 (2): 400–3. doi:10.1016/j.bmcl.2014.10.091. PMC 4297288. PMID 25510374.