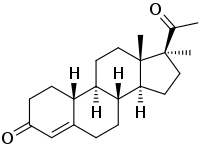
17α-Methyl-19-norprogesterone
17α-Methyl-19-norprogesterone (developmental code name H-3510), also known as 17α-methyl-19-norpregn-4-ene-3,20-dione, is a progestin which was never marketed.[1][2][3] It is a derivative of progesterone, and is the combined derivative of 17α-methylprogesterone and 19-norprogesterone.[1] The drug is the parent compound of a subgroup of the 19-norprogesterone group of progestins, which includes demegestone (the δ9 derivative), promegestone (the δ9 and 21-methyl derivative), and trimegestone (the δ9, 21-methyl, and 21-hydroxyl derivative).[4]
 | |
| Clinical data | |
|---|---|
| Other names | H-3510; 17α-Methyl-19-norpregn-4-ene-3,20-dione; (17β)-17-Acetyl-17-methylestr-4-en-3-one |
| Drug class | Progestin; Progestogen |
| Identifiers | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
- Gestronol (17α-hydroxy-19-norprogesterone)
References
- Weiss, M.J.; Schaub, R.E.; Allen, G.R.; Poletto, J.F.; Pidacks, V.; Conrow, R.B.; Coscia, C.J. (1964). "The formation of steroid enolate anions by reductive procedures". Tetrahedron. 20 (2): 357–372. doi:10.1016/S0040-4020(01)93223-5. ISSN 0040-4020.
- Raynaud JP, Philibert D, Azadian-Boulanger G (1974). "Progesterone-Progestin receptors". Basic Life Sci. 4 (PART A): 143–60. doi:10.1007/978-1-4684-2889-6_10. ISBN 978-1-4684-2891-9. PMID 4374925.
- Delettré J, Mornon JP, Lepicard G, Ojasoo T, Raynaud JP (January 1980). "Steroid flexibility and receptor specificity". J. Steroid Biochem. 13 (1): 45–59. doi:10.1016/0022-4731(80)90112-0. PMID 7382482.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.