Demegestone
Demegestone, sold under the brand name Lutionex, is a progestin medication which was previously used to treat luteal insufficiency but is now no longer marketed.[3][4][5][6][7] It is taken by mouth.[2][1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lutionex |
| Other names | Dimegestone; R-2453; RU-2453; 17α-Methyl-δ9-19-norprogesterone; 17α-Methyl-19-norpregna-4,9-diene-3,20-dione |
| Routes of administration | By mouth[1] |
| Drug class | Progestogen; Progestin |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | Good[2] |
| Metabolism | Hydroxylation, others[2] |
| Metabolites | • 21-Hydroxydemegestone[2] • Others[2] |
| Excretion | Urine[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.030.278 |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Demegestone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[6][2][8] It has no androgenic activity.[2]
Demegestone was first described in 1966 and was introduced for medical use in France in 1974.[3][4] It has only been marketed in France, and has since been discontinued in this country.[5][4]
Medical uses
Demegestone has been used to treat luteal insufficiency.[7] It has also been studied in combination with estrogens, such as moxestrol, as an oral contraceptive and treatment for infertility.[1][9][10]
Side effects
Pharmacology
Pharmacodynamics
Demegestone is a progestogen, and hence is an agonist of the progesterone receptor (PR).[6][8][2] It is a highly potent progestogen, showing 50 times the potency of progesterone in the Clauberg test.[2] The ovulation-inbhiting dosage of demegestone is 2.5 mg/day, while the endometrial transformation dosage is 100 mg per cycle.[11] The medication is devoid of androgenic activity,[2] and instead has some antiandrogenic activity.[12] Demegestone has low affinity for the glucocorticoid receptor.[13] In a particular bioassay, both demegestone and progesterone showed antiglucocorticoid rather than glucocorticoid activity.[14] The major metabolite of demegestone, a 21-hydroxylated metabolite, is a moderately potent progestogen (4 times the potency of progesterone) and a weak mineralocorticoid (2% of the potency of deoxycorticosterone).[2]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | |
|---|---|---|---|---|---|---|---|---|
| Demegestone | 230 | 1 | 0 | 5 | 1–2 | ? | ? | |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: [13][15][16][17] | ||||||||
Pharmacokinetics
Demegestone has good bioavailability.[2] The initial volume of distribution of demegestone is 31 L.[2] Demegestone is metabolized by hydroxylation at the C21, C1, C2, and C11 positions, which is eventually followed by A-ring aromatization after 1,2-dehydration.[2] The major metabolite of demegestone is a 21-hydroxy derivative.[2] The metabolic clearance rate of demegestone is 20 L/h.[2] Its biological half-lives are 2.39 and 0.24 hours with intravenous injection.[2] Demegestone and/or its metabolites are excreted, at least in part, in urine.[2]
Chemistry
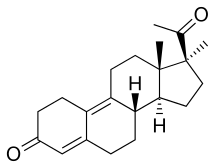
Demegestone, also known as 17α-methyl-δ9-19-norprogesterone or as 17α-methyl-19-norpregna-4,9-diene-3,20-dione, is a synthetic norpregnane steroid and a derivative of progesterone.[3][4][6] It is specifically a combined derivative of 17α-methylprogesterone and 19-norprogesterone, or of 17α-methyl-19-norprogesterone.[3][4][6] Related derivatives of 17α-methyl-19-norprogesterone include promegestone and trimegestone.[3][6]
History
Demegestone was first described in the literature in 1964 and was introduced for medical use in 1974 in France.[3][4] It was developed by Roussel Uclaf.[4]
Society and culture
Generic names
Demegestone is the generic name of the drug and its INN.[3] It is also known by its developmental code name R-2453 or RU-2453.[3]
References
- Iizuka R, Hayashi M, Kamouchi Y, Yamanaka K (1971). "Evaluation of a low-dose progestagen as a contraceptive". Nihon Funin Gakkai Zasshi. 16 (1): 68–82. PMID 12158578.
- Raynaud, J.P.; Cousty, C.; Salmon, J. (1974). "121. Metabolic studies of R2453, a highly potent progestin". Journal of Steroid Biochemistry. 5 (4): 324. doi:10.1016/0022-4731(74)90266-0. ISSN 0022-4731.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 356–. ISBN 978-1-4757-2085-3.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1215–. ISBN 978-0-8155-1856-3.
- http://www.micromedexsolutions.com/micromedex2/%5B%5D
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Pugeat M, Lejeune H, Dechaud H, Brébant C, Mallein R, Tourniaire J (1988). "[Luteal insufficiency and elevation of sex-binding proteins by demegestone]". Rev Fr Gynecol Obstet (in French). 83 (7–9): 495–8. PMID 3194612.
- Lee DL, Kollman PA, Marsh FJ, Wolff ME (September 1977). "Quantitative relationships between steroid structure and binding to putative progesterone receptors". J. Med. Chem. 20 (9): 1139–46. doi:10.1021/jm00219a006. PMID 926114.
- Hamada H, Nagao H, Toyoda H, Hayashi H, Akihiro L, & Kotaki S (1970). [Clinical observation on oral contraceptive effect by R-2453 (Abstracts of Papers Presented at Showa 44 in the field of gynecology]). Japanese Journal of Obstetrics and Gynecology-Acta Obstetrica et Gynaecologica Japonica, 22 (7), 753. https://ci.nii.ac.jp/naid/110002126113/
- Levrier, M. (1979, January). Treatment of Ovarian Sterility with Combined Moxestrol-Demegestone Preparation. In Journal De Gynecologie Obstetrique Et Biologie De La Reproduction (Vol. 8, No. 1, Pp. 89–89). 120 Blvd Saint-Germain, 75280 Paris 06, France: Masson Editeur.
- Rabe, T., Goeckenjan, M., Ahrendt, H. J., Crosignani, P. G., Dinger, J. C., Mueck, A. O., ... & Strowitzki, T. (2011). Oral Contraceptive Pills: Combinations, Dosages and the Rationale behind 50 Years or Oral Hormonal Contraceptive Development. Journal für Reproduktionsmedizin und Endokrinologie-Journal of Reproductive Medicine and Endocrinology, 8(1), 58-129. http://www.kup.at/kup/pdf/10166.pdf
- Raynaud, J. P.; Ojasoo, T.; Labrie, F. (1981). "Steroid hormones—agonists and antagonists". Mechanisms of Steroid Action. pp. 145–158. doi:10.1007/978-1-349-81345-2_11. ISBN 978-1-349-81347-6.
- Delettré J, Mornon JP, Lepicard G, Ojasoo T, Raynaud JP (January 1980). "Steroid flexibility and receptor specificity". J. Steroid Biochem. 13 (1): 45–59. doi:10.1016/0022-4731(80)90112-0. PMID 7382482.
- Dausse JP, Duval D, Meyer P, Gaignault JC, Marchandeau C, Raynaud JP (September 1977). "The relationship between glucocorticoid structure and effects upon thymocytes". Mol. Pharmacol. 13 (5): 948–55. PMID 895725.
- Raynaud JP, Bouton MM, Moguilewsky M, Ojasoo T, Philibert D, Beck G, Labrie F, Mornon JP (January 1980). "Steroid hormone receptors and pharmacology". J. Steroid Biochem. 12: 143–57. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
- Ojasoo T, Raynaud JP, Doé JC (January 1994). "Affiliations among steroid receptors as revealed by multivariate analysis of steroid binding data". J. Steroid Biochem. Mol. Biol. 48 (1): 31–46. doi:10.1016/0960-0760(94)90248-8. PMID 8136304. S2CID 21336380.
- Ojasoo T, Raynaud JP (November 1978). "Unique steroid congeners for receptor studies". Cancer Res. 38 (11 Pt 2): 4186–98. PMID 359134.