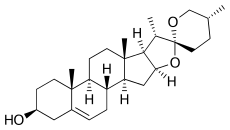
Diosgenin
Diosgenin, a phytosteroid sapogenin, is the product of hydrolysis by acids, strong bases, or enzymes of saponins, extracted from the tubers of Dioscorea wild yam, such as the Kokoro. The sugar-free (aglycone) product of such hydrolysis, diosgenin is used for the commercial synthesis of cortisone, pregnenolone, progesterone, and other steroid products.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3β,25R)-spirost-5-en-3-ol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.396 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H42O3 | |
| Molar mass | 414.630 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sources
It is present in detectable amounts in Costus speciosus, Smilax menispermoidea, species of Paris, Aletris, Trigonella, and Trillium, and in extractable amounts many species of Dioscorea – D. althaeoides, colletti, composita,[1] floribunda, futschauensis, gracillima, hispida, hypoglauca, mexicana,[2] nipponica, panthaica, parviflora, septemloba,Helicteres isora and zingiberensis.[3]
Industrial uses
Diosgenin is a precursor for several hormones, starting with the Marker degradation process, which includes synthesis of progesterone.[4] The process was used in the early manufacturing of combined oral contraceptive pills.[5]
References
- "Dioscorea composita". Germplasm Resources Information Network (GRIN). Agricultural Research Service (ARS), United States Department of Agriculture (USDA). Retrieved 2008-09-14.
- "Dioscorea mexicana". Germplasm Resources Information Network (GRIN). Agricultural Research Service (ARS), United States Department of Agriculture (USDA). Retrieved 2008-09-14.
- "2950 Diosgenin". Retrieved 2007-05-29.
- Marker RE, Krueger J (1940). "Sterols. CXII. Sapogenins. XLI. The Preparation of Trillin and its Conversion to Progesterone". J. Am. Chem. Soc. 62 (12): 3349–3350. doi:10.1021/ja01869a023.
- Djerassi C (December 1992). "Steroid research at Syntex: "the pill" and cortisone". Steroids. 57 (12): 631–41. doi:10.1016/0039-128X(92)90016-3. PMID 1481227.