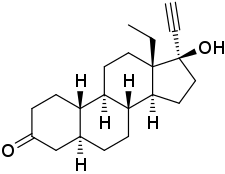
5α-Dihydrolevonorgestrel
5α-Dihydrolevonorgestrel (5α-DHLNG) is an active metabolite of the progestin levonorgestrel which is formed by 5α-reductase.[1][2] It has about one-third of the affinity of levonorgestrel for the progesterone receptor.[1] In contrast to levonorgestrel, the compound has both progestogenic and antiprogestogenic activity, and hence has a selective progesterone receptor modulator-like profile of activity.[3][4] This is analogous to the case of norethisterone and 5α-dihydronorethisterone.[3][5] In addition to the progesterone receptor, 5α-DHLNG interacts with the androgen receptor.[6] It has similar affinity for the androgen receptor relative to levonorgestrel (34.3% of that of metribolone for levonorgestrel and 38.0% of that of metribolone for 5α-DHLNG), and has androgenic effects similarly to levonorgestrel and testosterone.[6] 5α-DHLNG is further transformed into 3α,5α- and 3β,5α-THLNG, which bind weakly to the estrogen receptor (0.4 to 2.4% of the RBA of E2) and have weak estrogenic activity.[7][8][4] These metabolites are considered to be responsible for the weak estrogenic activity of high doses of levonorgestrel.[8][4]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Levonorgestrel | 150–162 | 34a, 45 | 0 | 1–8 | 17–75 | 50 | 0 |
| 5α-Dihydrolevonorgestrel | 50 | 38a | 0 | ? | ? | ? | ? |
| 3α,5α-Tetrahydrolevonorgestrel | ? | ? | 0.4 | ? | ? | ? | ? |
| 3β,5α-Tetrahydrolevonorgestrel | ? | ? | 2.4 | ? | ? | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone (a = mibolerone) for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: See template. | |||||||
 | |
| Clinical data | |
|---|---|
| Other names | 5α-Dihydrolevonorgestrel; 5α-DHLNG; 5α-LNG |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (2008). "Classification and pharmacology of progestins". Maturitas. 61 (1–2): 171–80. doi:10.1016/j.maturitas.2008.11.013. PMID 19434889.
- Vij U, Murugesan K, Kalita JC, Farooq A (February 1989). "Interaction of antiprogestins with progesterone receptors in rat uterus". J. Steroid Biochem. 32 (2): 279–82. doi:10.1016/0022-4731(89)90264-1. PMID 2921869.
- García-Becerra R, Borja-Cacho E, Cooney AJ, Jackson KJ, Lemus AE, Pérez-Palacios G, Larrea F (November 2002). "The intrinsic transcriptional estrogenic activity of a non-phenolic derivative of levonorgestrel is mediated via the estrogen receptor-alpha". J. Steroid Biochem. Mol. Biol. 82 (4–5): 333–41. doi:10.1016/s0960-0760(02)00192-9. PMID 12589940. S2CID 24204715.
- Chu YH, Li QA, Zhao ZF, Zhou YP, Cao DC (1985). "[Antiprogestational action of 5 alpha-dihydronorethisterone]". Zhongguo Yao Li Xue Bao (in Chinese). 6 (2): 125–9. PMID 2934946.
- Cabeza M, Vilchis F, Lemus AE, Díaz de León L, Pérez-Palacios G (September 1995). "Molecular interactions of levonorgestrel and its 5 alpha-reduced derivative with androgen receptors in hamster flanking organs". Steroids. 60 (9): 630–5. doi:10.1016/0039-128X(95)00075-2. PMID 8545853. S2CID 20869899.
- Khan FS, Fotherby K (April 1979). "In vitro metabolism of 17 alpha-ethynylsteroids". J. Steroid Biochem. 10 (4): 437–42. doi:10.1016/0022-4731(79)90332-7. PMID 449320.
- Santillán R, Pérez-Palacios G, Reyes M, Damián-Matsumura P, García GA, Grillasca I, Lemus AE (September 2001). "Assessment of the oestrogenic activity of the contraceptive progestin levonorgestrel and its non-phenolic metabolites". Eur. J. Pharmacol. 427 (2): 167–74. doi:10.1016/S0014-2999(01)01263-8. PMID 11557270.