11-Dehydroprogesterone
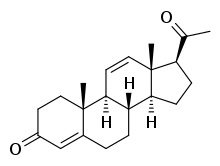
11-Dehydroprogesterone, also known as pregna-4,11-diene-3,20-dione, is a steroidal progestin that was never marketed.[1][2][3] It was found to be 2- to 3-fold as potent as progesterone as a progestogen in animal bioassays,[1][4] although other studies found them to be equivalent in potency.[2] 11-Dehydroprogesterone has been studied in women.[5] It was discovered in the 1930s or 1940s and was one of the earliest synthetic progestogens.[3]
 | |
| Clinical data | |
|---|---|
| Other names | Pregna-4,11-diene-3,20-dione |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- ZARROW MX, PETERS LE, CALDWELL AL (1958). "Comparative potency of several progestogenic compounds in a battery of different biological tests". Ann. N. Y. Acad. Sci. 71 (5): 532–41. doi:10.1111/j.1749-6632.1958.tb46783.x. PMID 13583809.
- ZARROW MX, NEHER GM, LAZOWASEM EA, SALHANICK HA (1957). "Biological activity of certain progesterone-like compounds as determined by the Hooker-Forbes bioassay". J. Clin. Endocrinol. Metab. 17 (5): 658–66. doi:10.1210/jcem-17-5-658. PMID 13416376.
- Pfiffner, J J; Kamm, O (1942). "The Chemistry of the Hormones". Annual Review of Biochemistry. 11 (1): 283–308. doi:10.1146/annurev.bi.11.070142.001435. ISSN 0066-4154.
- TULLNER WW, HERTZ R (1953). "High progestational activity of 19-norprogesterone". Endocrinology. 52 (3): 359–61. doi:10.1210/endo-52-3-359. PMID 13033848.
- FERIN J (1950). "[Effects of 11-dehydroprogesterone in women]". Ann. Endocrinol. Paris. 11 (2): 179–82. PMID 14799957. (in Undetermined Language)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.