Norgestrienone
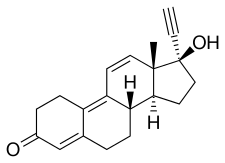
Norgestrienone, sold under the brand names Ogyline, Planor, and Miniplanor, is a progestin medication which has been used in birth control pills, sometimes in combination with ethinylestradiol.[1][2][3][4][5] It was developed by Roussel Uclaf and has been registered for use only in France.[4][5][6] Under the brand name Planor, it has been marketed in France as 2 mg norgestrienone and 50 μg ethinylestradiol tablets.[7] It is taken by mouth.[5]
 | |
| Clinical data | |
|---|---|
| Trade names | Ogyline, Planor, Miniplanor |
| Other names | RU-2010; A-301; 17α-Ethynyltrienolone; 17α-Ethynyltrenbolone; Δ9,11-Norethisterone; 17α-Ethynylestra-4,9,11-trien-17β-ol-3-one |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Androgen; Anabolic steroid |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.544 |
| Chemical and physical data | |
| Formula | C20H22O2 |
| Molar mass | 294.394 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Norgestrienone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[8] It has some androgenic activity.[9][10][11][12]
Norgestrienone was first described in the literature in 1965.[10] It is sometimes referred to as a "second-generation" progestin.[13] Norgestrienone is no longer available.[14]
Medical uses
Norgestrienone was used in hormonal contraception to prevent pregnancy.[2][7] It has typically been used as an oral contraceptive at a dosage of 2 mg/day in combination with ethinylestradiol and 350 µ/day when used alone.[5]
Side effects
Pharmacology
Pharmacodynamics
Norgestrienone has been found to possess similar affinity for the progesterone receptor and androgen receptor,[8] and in accordance, has some androgenic activity.[9][10][11][12] The androgenic activity of norgestrienone is greater than that of other 19-nortestosterone derivatives due to the presence of the C9(11) double bond, which enhances said activity.[12] The ratio of progestogenic to androgenic activity appears to be much lower for norgestrienone that it is for other 19-nortestosterone progestins such as norethisterone and levonorgestrel.[15][16][17][18] Gestrinone, the 18-methyl analogue of norgestrienone, has even greater androgenic activity than norgestrienone, as this modification increases androgenic activity similarly.[12]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | |
|---|---|---|---|---|---|---|---|---|
| Norethisterone | 155–156 | 43–45 | <0.1 | 2.7–2.8 | 0.2 | ? | ? | |
| Norgestrienone | 63–65 | 70 | <0.1 | 11 | 1.8 | ? | ? | |
| Levonorgestrel | 170 | 84–87 | <0.1 | 14 | 0.6–0.9 | ? | ? | |
| Gestrinone | 75–76 | 83–85 | <0.1, 3–10 | 77 | 3.2 | ? | ? | |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: [15][16][17][18] | ||||||||
Pharmacokinetics
The metabolism of norgestrienone in humans has been studied.[19]
Chemistry
Norgestrienone, also known as 17α-ethynyl-19-nor-δ9,11-testosterone or as 17α-ethynylestra-4,9,11-trien-17β-ol-3-one, as well as δ9,11-norethisterone or 17α-ethynyltrienolone (17α-ethynyltrenbolone), is a synthetic estrane steroid and a derivative of testosterone and 19-nortestosterone.[1][4][20] It is structurally related to the anabolic steroid trenbolone (19-nor-δ9,11-testosterone; the non-17α-ethynylated analogue of norgestrienone), the progestogenic and androgenic steroid gestrinone (the 13β-ethyl variant or 18-methyl derivative of norgestrienone), and the anabolic steroid tetrahydrogestrinone (the 18-methyl and 17α-ethyl variant of norgestrienone).[1][4][21]
History
Norgestrienone was first described in the literature in 1965.[10] It is sometimes referred to as a "second-generation" progestin based on its time of introduction.[13]
Society and culture
Generic names
Norgestrienone is the generic name of the drug and its INN.[1][2][4] It is also known by its developmental code names RU-2010 and A-301.[1][2][4]
Research
Norgestrienone has been studied for use in male hormonal contraception.[23]
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 887–. ISBN 978-1-4757-2085-3.
- Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 202–. ISBN 978-94-011-4439-1.
- Diaz S, Pavez M, Quinteros E, Diaz J, Robertson DN, Croxatto HB (October 1978). "Clinical trial with subdermal implants containing norgestrienone". Contraception. 18 (4): 429–40. doi:10.1016/0010-7824(78)90027-6. PMID 720075.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 751–. ISBN 978-3-88763-075-1.
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2122. ISBN 978-0-85369-840-1.
Norgestrienone is a progestogen structurally related to norethisterone that has been used as an oral contraceptive. Typical doses have been 2 mg daily with an oestrogen, and 350 micrograms daily when used alone.
- McGuire JL (2000). Pharmaceuticals, 4 Volume Set. Wiley. p. 1580,1599. ISBN 978-3-527-29874-7.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 455–. ISBN 978-92-832-1291-1.
- Loughney DA, Schwender CF (December 1992). "A comparison of progestin and androgen receptor binding using the CoMFA technique". Journal of Computer-Aided Molecular Design. 6 (6): 569–81. doi:10.1007/bf00126215. PMID 1291626. S2CID 22004130.
- Axelrod J (1 January 1982). Biochemical Actions of Hormones. Academic Press. ISBN 978-0-12-452809-3.
- Lauritzen C, Studd JW (22 June 2005). Current Management of the Menopause. CRC Press. pp. 45–. ISBN 978-0-203-48612-2.
- Di Giulio RT, Monosson E (6 December 2012). Interconnections Between Human and Ecosystem Health. Springer Science & Business Media. pp. 60–. ISBN 978-94-009-1523-7.
- Rozenbaum H (March 1982). "Relationships between chemical structure and biological properties of progestogens". Am. J. Obstet. Gynecol. 142 (6 Pt 2): 719–24. doi:10.1016/S0002-9378(16)32477-2. PMID 7065053.
- Weiss G (February 1999). "Risk of venous thromboembolism with third-generation oral contraceptives: A review". Am. J. Obstet. Gynecol. 180 (2 Pt 2): 295–301. doi:10.1016/S0002-9378(99)70721-0. PMID 9988833.
- http://www.micromedexsolutions.com/micromedex2/%5B%5D
- Delettré J, Mornon JP, Lepicard G, Ojasoo T, Raynaud JP (January 1980). "Steroid flexibility and receptor specificity". J. Steroid Biochem. 13 (1): 45–59. doi:10.1016/0022-4731(80)90112-0. PMID 7382482.
- Raynaud JP, Bouton MM, Moguilewsky M, Ojasoo T, Philibert D, Beck G, Labrie F, Mornon JP (January 1980). "Steroid hormone receptors and pharmacology". J. Steroid Biochem. 12: 143–57. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
- Ojasoo T, Raynaud JP, Doé JC (January 1994). "Affiliations among steroid receptors as revealed by multivariate analysis of steroid binding data". J. Steroid Biochem. Mol. Biol. 48 (1): 31–46. doi:10.1016/0960-0760(94)90248-8. PMID 8136304. S2CID 21336380.
- Raynaud, J.P.; Ojasoo, T.; Bouton, M.M.; Philibert, D. (1979). "Receptor Binding as a Tool in the Development of New Bioactive Steroids". Drug Design. pp. 169–214. doi:10.1016/B978-0-12-060308-4.50010-X. ISBN 9781483216102.
- Raynaud, J. P. (1971). Metabolism of contraceptive steroids in man. https://www.popline.org/node/480947
- Lavery JP, Sanfilippo JS (6 December 2012). Pediatric and Adolescent Obstetrics and Gynecology. Springer Science & Business Media. pp. 236–. ISBN 978-1-4612-5064-7.
- Gomel V, Brill A (27 September 2010). Reconstructive and Reproductive Surgery in Gynecology. CRC Press. pp. 90–. ISBN 978-1-84184-757-3.
- Daniel Lednicer (4 March 2009). Strategies for Organic Drug Synthesis and Design. John Wiley & Sons. pp. 134–. ISBN 978-0-470-39959-0.
- Scheater, S. B. (1978). The use of progestins and androgens as a male contraceptive. https://www.popline.org/node/443605