Flumedroxone acetate
Flumedroxone acetate, sold under the brand names Demigran and Leomigran, is a progestin medication which is or has been used as an antimigraine agent.[2][3][4] It is taken by mouth.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Demigran, Leomigran |
| Other names | WG-537; 6α-(Trifluoromethyl)-17α-acetoxyprogesterone; 6α-(Trifluoromethyl)-17α-acetoxypregn-4-ene-3,20-dione |
| Routes of administration | By mouth[1] |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.012.343 |
| Chemical and physical data | |
| Formula | C24H31F3O4 |
| Molar mass | 440.503 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Medical uses
Flumedroxone acetate has been assessed in over 1,000 patients for the treatment of migraine, with effectiveness ranging from excellent to less than that of the reference antimigraine drug methysergide.[5] Other progestogens including medroxyprogesterone acetate, lynestrenol, allylestrenol, dydrogesterone, and normethandrone have also been found to be effective for migraine in a high percentage of women.[5]
Side effects
In accordance with its progestogenic activity, flumedroxone acetate produces menstrual irregularities, namely polymenorrhea, and breast tension as side effects in women.[5][6][7]
Pharmacology
Pharmacodynamics
Flumedroxone acetate is said to have weak or slight progestogenic activity without other hormonal activity, including no estrogenic, antiestrogenic, androgenic, anabolic, or glucocorticoid activity.[5][1]
Chemistry
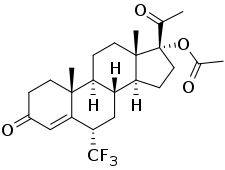
Flumedroxone acetate, also known as 6α-(trifluoromethyl)-17α-acetoxyprogesterone or as 6α-(trifluoromethyl)-17α-acetoxypregn-4-ene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone.[2] It is specifically a derivative of 17α-hydroxyprogesterone with a trifluoromethyl group at the C6α position and an acetate ester attached to the C17α hydroxyl group.[3] The medication is the C17α acetate ester of flumedroxone (6α-(trifluoromethyl)-17α-hydroxyprogesterone) and the C6α trifluoromethyl derivative of hydroxyprogesterone acetate (17α-acetoxyprogesterone).[2]
History
Flumedroxone acetate was introduced for medical use in the 1960s.[4]
Society and culture
Generic names
Flumedroxone is the INN and BAN of the free alcohol form of the drug, flumedroxone.[2] Flumedroxone acetate is also known by its developmental code name WG-537.[2]
References
- Hartwig Heyck (1981). Headache and facial pain: differential diagnosis, pathogenesis, treatment. Year Book Medical. ISBN 978-3-13-589501-7.
Lundberg (1969) recommended the oral application of a steroid (flumedroxone) which has only a weak progesterone effect and does not display any other gonadotropic effects.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 560–. ISBN 978-1-4757-2085-3.
- Jean-Pierre Bégué; Daniele Bonnet-Delpon (2 June 2008). Bioorganic and Medicinal Chemistry of Fluorine. John Wiley & Sons. pp. 329–. ISBN 978-0-470-28187-1.
- Hudgson P, Foster JB, Newell DJ (1967). "Controlled trial of demigran in the prophylaxis of migraine". Br Med J. 2 (5544): 91–3. doi:10.1136/bmj.2.5544.91. PMC 1841258. PMID 6020856.
- Volker Pfaffenrath; Per-Olov Lundberg; Ottar Sjaastad (6 December 2012). Updating in Headache. Springer Science & Business Media. pp. 229–. ISBN 978-3-642-88581-5.
- Bradley WG, Hudgson P, Foster JB, Newell DJ (1968). "Double-blind controlled trial of a micronized preparation of flumedroxone (Demigran) in prophylaxis of migraine". Br Med J. 3 (5617): 531–3. doi:10.1136/bmj.3.5617.531. PMC 1986487. PMID 4877801.
- Lundberg PO (1969). "Prophylactic treatment of migraine with flumedroxone". Acta Neurol. Scand. 45 (3): 309–26. doi:10.1111/j.1600-0404.1969.tb01243.x. PMID 5817457. S2CID 9835328.