Carbon capture and utilization
Carbon capture and utilization (CCU) is the process of capturing carbon dioxide (CO2) to be recycled for further usage.[1] Carbon capture and utilization may offer a response to the global challenge of significantly reducing greenhouse gas emissions from major stationary (industrial) emitters.[2] CCU differs from Carbon Capture and Storage (CCS) in that CCU does not aim nor result in permanent geological storage of carbon dioxide. Instead, CCU aims to convert the captured carbon dioxide into more valuable substances or products; such as plastics, concrete or biofuel; while retaining the carbon neutrality of the production processes.
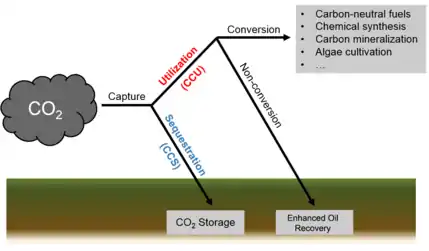
Captured CO2 can be converted to several products: one group being hydrocarbons, such as methanol, to use as biofuels and other alternative and renewable sources of energy. Other commercial products include plastics, concrete and reactants for various chemical synthesis.[3]
Although CCU does not result in a net carbon positive to the atmosphere, there are several important considerations to be taken into account. The energy requirement for the additional processing of new products should not exceed the amount of energy released from burning fuel as the process will require more fuel. Because CO2 is a thermodynamically stable form of carbon manufacturing products from it is energy intensive.[4] In addition, concerns on the scale of CCU is a major argument against investing in CCU. The availability of other raw materials to create a product should also be considered before investing in CCU.
Considering the different potential options for capture and utilization, research suggests that those involving chemicals, fuels and microalgae have limited potential for CO
2 removal, while those that involve construction materials and agricultural use can be more effective.[5]
The profitability of CCU depends partly on the carbon price of CO2 being released into the atmosphere. Using captured CO2 to create useful commercial products could make carbon capture financially viable.[6]
Sources of carbon
CO2 is typically captured from fixed point sources such as power plants and factories.[4] CO2 captured from these exhaust stream itself varies in concentration. A typical coal power plant will have 10-12% CO2 concentration in its flue gas exhaust stream.[7] A biofuel refinery produces a high purity (99%) of CO2 with small amount of impurities such as water and ethanol.[7] The separation process itself can be performed through separation processes such as absorption, adsorption, or membranes.
Another possible source of capture in CCU process involves the use of plantation. The idea originates from the observation in the Keeling curve that the CO2 level in the atmosphere undergoes annual variation of approximately 5 ppm (parts per million), which is attributed to the seasonal change of vegetation and difference in land mass between the northern and southern hemisphere.[8][9] However, the CO2 sequestered by the plants will be returned to the atmosphere when the plants died. Thus, it is proposed to plant crops with C4 photosynthesis, given its rapid growth and high carbon capture rate, and then to process the biomass for applications such as biochar that will be stored in the soil permanently.[10]
Examples of technology and application
| Part of a series about |
| Sustainable energy |
|---|
 |
| Overview |
| Energy conservation |
| Renewable energy |
| Sustainable transport |
|
CO2 electrolysis
CO2 electroreduction to a variety of value-added products has been under development for many years. Some major targets are formate, oxalate, and methanol, as electrochemical formation of these products from CO2 would constitute a very environmentally sustainable practice.[11] For example, CO2 can be captured and converted to carbon-neutral fuels in an aqueous catalysis process.[12][13][14] It is possible to convert CO2 in this way directly to ethanol, which can then be upgraded to gasoline and jet fuel-[15]
Carbon-neutral fuel
A carbon-neutral fuel can be synthesized by using the captured CO2 from the atmosphere as the main hydrocarbon source. The fuel is then combusted and CO2, as the byproduct of the combustion process, is released back into the air. In this process, there is no net carbon dioxide released or removed from the atmosphere, hence the name carbon-neutral fuel. An example of the technology include biofuel from microalgae as discussed below.
Methanol fuel
A proven process to produce a hydrocarbon is to make methanol. Methanol is easily synthesized from CO
2 and H2. Based on this fact the idea of a methanol economy was born.
Methanol, or methyl alcohol, is the simplest member of the family of alcohol organic compound with a chemical formula of CH3OH. Methanol fuel can be manufactured using the captured carbon dioxide while performing the production with renewable energy. Consequently, methanol fuel has been considered as an alternative to fossil fuels in power generation for achieving a carbon-neutral sustainability.[16][17] Carbon Recycling International, a company with production facility in Grindavik, Iceland, markets such Emission-to-Liquid renewable high octane methanol fuel with current 4,000 metric ton/year production capacity.[18]
Chemical synthesis
As a highly desirable C1 (one-carbon) chemical feedstock, CO2 captured previously can be converted to a diverse range of products. Some of these products include: polycarbonates (via Zinc based catalyst) or other organic products such as acetic acid,[19] urea,[19] and PVC.[20] Currently 75% (112 million tons) of urea production, 2% (2 million tons) of methanol production, 43% (30 thousand tons) of salicylic acid production, and 50% (40 thousand tons) of cyclic carbonates production utilize CO2 as a feedstock.[21] Chemical synthesis is not a permanent storage/utilization of CO2, as aliphatic (straight chain) compounds may degrade and release CO2 back to the atmosphere as early as 6 months.[20]
Novomer is a chemicals company working on a zinc-based catalyst for production of polyethylene carbonate (PEC) and polypropylene carbonate (PPC) feedstock. A March 2011 report by Global CCS Institute foresaw annual production potential of 22.5 MtCO2/yr. They have received funding from multiple sources such as the Department of Energy (DOE) ($2.6 million) and NSF ($400,000) to achieve commercialization as well as converting their production process from a batch process to a continuous process.[20]
Enhanced Oil Recovery (EOR)
In EOR, the captured CO2 is injected into depleted oil fields with the goal to increase the amount of oil to be extracted by the wells. This method is proven to increase oil output by 5-40%.[20] The scale of CO2 utilization through this technologies ranges from 30-300 MtCO2/yr. It is a permanent and mature technology in CCU. The biggest market driver for EOR is the heavy reliance on oil. In United States, some of the additional market drivers include: tax revenue for foreign oil as well as the presence of carbon tax credits.
Carbon mineralization
Carbon dioxide from sources such as flue gas are reacted with minerals such as magnesium oxide and calcium oxide to form stable solid carbonates. Sources of minerals include brine and waste industrial minerals. The carbonates can then be used for construction, consumer products, and as an alternative for carbon capture and sequestration (CCS). The scale of this technology may reach more than 300 Mt of CO2 removed per year. 0.5 tonnes of CO2 is removed from the air for every tonne of mineral carbonate produced. However, it needs 1–5 years to commercialization as the technology is not mature yet.
The company Calera proposed a way to mineralize CO2 through the CMAP process. This process involves precipitating a carbonate slurry from a mixture of water, solid minerals, and flue gas. The products are concentrated pumpable carbonate suspension, fresh water, and CO2-free flue gas.
Benefits of this process includes the production of fresh water and that the CO2 used does not require separation or compression. A barrier of this technology is, however, the competition with existing cement industries.
Biofuel from microalgae

A study has suggested that microalgae can be used as an alternative source of energy.[22] A pond of microalgae is fed with a source of carbon dioxide such as flue gas and the microalgae is then let to proliferate. The algae is then harvested and the biomass obtained is then converted to biofuel. 1.8 tonnes of CO2 is removed from the air per 1 metric tonne of dry algal biomass produced. This number actually varies depending on the species. The scale of this technology may reach more than 300Mt of CO2 removed per year. The CO2 captured will be stored non-permanently as the biofuel produced will then be combusted and the CO2 will be released back into the air. However, the CO2 released was first captured from the atmosphere and releasing it back into the air makes the fuel a carbon-neutral fuel. This technology is not mature yet.[23]
Dead algae can sink to the bottom of the lake and result in permanent storage. However, the algae needs a large area of pond and sunlight all year round to remove CO2 all year round. Furthermore, the pond environment needs to be controlled since the algae needs to live in a specific condition. There are concerns about how the algae-filled pond might affect the environment and ecosystem around it.
Agriculture
An approach that is also proposed as a climate change mitigation effort is to perform plant-based carbon capture.[24] The resulting biomass can then be used for fuel, while the biochar byproduct is then utilized for applications in agriculture as soil-enhancer. Cool Planet is a private company with an R&D plant in Camarillo, California, performed development of biochar for agricultural applications and claimed that their product can increase crops yield by 12.3% and three-fold return of investment via improvement of soil health and nutrient retention.[25] However, the claims on the efficacy of plant-based carbon capture for climate change mitigation has received a fair amount of skepticism.[26]
Environmental impacts
16 life cycle environmental impact analyses have been done to assess the impacts of four main CCU technologies against conventional CCS: Chemical synthesis, carbon mineralization, biodiesel production, as well as Enhanced Oil Recovery (EOR). These technologies were assessed based on 10 Life-cycle assessment (LCA) impacts such as: acidification potential, eutrophication potential, global warming potential, and ozone depletion potential. The conclusion from the 16 different models was that chemical synthesis has the highest global warming potential (216 times that of CCS) while enhanced oil recovery has the least global warming potential (1.8 times that of CCS).[1]
See also
- Carbon capture and storage
- Climate change mitigation
- Carbon neutral fuel
- Carbon sequestration
- Greenhouse gas removal
- List of energy topics
- Low-carbon economy
- Solar Foods Ltd.
References
- Cuéllar-Franca, Rosa M.; Azapagic, Adisa (2015-03-01). "Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts". Journal of CO2 Utilization. 9: 82–102. doi:10.1016/j.jcou.2014.12.001. ISSN 2212-9820.
- "Carbon Capture". Center for Climate and Energy Solutions. Retrieved 2020-04-22.
- Dibenedetto, Angela; Angelini, Antonella; Stufano, Paolo (2014-03-01). "Use of carbon dioxide as feedstock for chemicals and fuels: homogeneous and heterogeneous catalysis". Journal of Chemical Technology & Biotechnology. 89 (3): 334–353. doi:10.1002/jctb.4229. ISSN 1097-4660.
- Smit, Berend; Reimer, Jeffrey A; Oldenburg, Curtis M; Bourg, Ian C (2013-06-18). Introduction to Carbon Capture and Sequestration. The Berkeley Lectures on Energy. Imperial College Press. doi:10.1142/p911. ISBN 9781783263271.
- Hepburn, Cameron; Adlen, Ella; Beddington, John; Carter, Emily A.; Fuss, Sabine; Mac Dowell, Niall; Minx, Jan C.; Smith, Pete; Williams, Charlotte K. (6 November 2019). "The technological and economic prospects for CO2 utilization and removal". Nature. 575 (7781): 87–97. doi:10.1038/s41586-019-1681-6. PMID 31695213.
- Biniek, Krysta; Davies, Ryan; Henderson, Kimberly. "Why commercial use could be the future of carbon capture | McKinsey". mckinsey.com. Retrieved 12 January 2018.
- Xu, Yixiang; Isom, Loren; Hanna, Milford A. (2010-05-01). "Adding value to carbon dioxide from ethanol fermentations". Bioresource Technology. 101 (10): 3311–3319. doi:10.1016/j.biortech.2010.01.006. ISSN 0960-8524. PMID 20110166.
- Keeling, Charles (June 1960). "The concentration and isotopic abundances of carbon dioxide in the atmosphere" (PDF). Tellus. 12 (2): 200–203. doi:10.3402/tellusa.v12i2.9366.
- Keeling, Charles; et al. (1976). "Atmospheric carbon dioxide variations at Mauna Loa Observatory, Hawaii". Tellus. 28 (6): 538–551. doi:10.3402/tellusa.v28i6.11322.
- X, the moonshot factory, We Solve for X: Mike Cheiky on negative carbon liquid fuels, retrieved 2018-12-08
- Robert Francke; Benjamin Schille; Michael Roemelt (2018). "Homogeneously Catalyzed Electroreduction of Carbon Dioxide—Methods, Mechanisms, and Catalysts". Chem. Rev. 118 (9): 4631–4701. doi:10.1021/acs.chemrev.7b00459. PMID 29319300.
- "Nano-spike catalysts convert carbon dioxide directly into ethanol | ORNL". www.ornl.gov. Retrieved 2020-01-23.
- "Copper Catalyst Yields High Efficiency CO2-to-Fuels Conversion | Research UC Berkeley". vcresearch.berkeley.edu. Retrieved 2020-01-23.
- Dimmer, Olivia. "Turning CO₂ into Ethanol: Researchers Light the Way for Sustainable Energy Production". news.iit.edu. Retrieved 2020-01-23.
- "Ethanol Producer Magazine – The Latest News and Data About Ethanol Production". www.ethanolproducer.com. Retrieved 2020-01-23.
- Olah, George A. (2005-04-29). "Beyond Oil and Gas: The Methanol Economy". Angewandte Chemie International Edition. 44 (18): 2636–2639. doi:10.1002/anie.200462121. ISSN 1521-3773. PMID 15800867.
- Hagen, David (27 December 1978). "METHANOL: ITS SYNTHESIS, USE AS A FUEL, ECONOMICS, AND HAZARDS". Energy Research and Development Administration. Retrieved 7 December 2018.
- "Vulcanol". CRI - Carbon Recycling International. Retrieved 2018-12-08.
- Council, National Research (2001-06-27). Carbon Management: Implications for R & D in the Chemical Sciences and Technology (A Workshop Report to the Chemical Sciences Roundtable). doi:10.17226/10153. ISBN 9780309075732. PMID 20669488.
- "Accelerating the uptake of CCS: industrial use of captured carbon dioxide" (PDF). globalccsinstitute.com. Global CCS Institute. March 2011. Retrieved 3 October 2020.
- Erdogan Alper; Ozge Yuksel Orhan (2017). "CO2 utilization: Developments in conversion processes". Petroleum. 3: 109–126. doi:10.1016/j.petlm.2016.11.003.
- Oncel, Suphi S. (2013-10-01). "Microalgae for a macroenergy world". Renewable and Sustainable Energy Reviews. 26: 241–264. doi:10.1016/j.rser.2013.05.059. ISSN 1364-0321.
- https://www.chemengonline.com/mechanical-co2-sequestration-improves-algae-production/
- Matovic, Darko (2011-04-01). "Biochar as a viable carbon sequestration option: Global and Canadian perspective". Energy. 36 (4): 2011–2016. doi:10.1016/j.energy.2010.09.031. ISSN 0360-5442.
- "Cool Planet Completes 100th Independent Trial of Cool Terra®" (PDF). Cool Planet. 19 March 2018.
- Popper, Ben (2014-04-14). "The inventor of everything". The Verge. Retrieved 2018-12-08.
- "Demonstration projects | Global CCS Institute". hub.globalccsinstitute.com. Retrieved 2018-12-07.
Further reading
- CCUS in Clean Energy Transitions. International Energy Agency (published September 2020). 2020.
- Smit, Berend; Jeffrey A Reimer; Curtis M Oldenburg; Ian C Bourg (2014). Introduction to Carbon Capture and Sequestration. The Berkeley Lectures on Energy. 1. doi:10.1142/p911. ISBN 978-1-78326-327-1., Imperial College Press, ISBN 978-1-78326-327-1
- SAPEA, Science Advice for Policy by European Academies (2018). Novel carbon capture and utilisation technologies: research and climate aspects.https://www.sapea.info/ccu/: SAPEA, Science Advice for Policy by European Academies. ISBN 978-3-9819415-5-5. doi: 10.26356/CARBONCAPTURE
- New route to carbon-neutral fuels from carbon dioxide discovered by Stanford-DTU team
