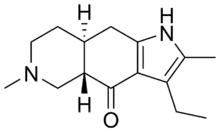
Piquindone
Piquindone (Ro 22-1319) is an atypical antipsychotic with a tricyclic structure that was developed in the 1980s but was never marketed.[1][2][3] It acts as a selective D2 receptor antagonist,[4][5][6] though based on its effects profile its selectivity may be considered controversial. Unlike most other D2 receptor ligands, piquindone displays Na+-dependent binding, a property it shares with tropapride, zetidoline, and metoclopramide.[7]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
| 3D model (JSmol) | |
| |
In clinical trials piquindone was found to possess moderate efficacy in treating positive symptoms of schizophrenia, and notably, was also modestly effective for negative symptoms, though this was just under statistical significance.[1] Additionally, relative to haloperidol, it was found to possesses significantly fewer extrapyramidal symptoms and had a much lower propensity for inducing tardive dyskinesia, indicating its atypical nature.[1][3] In addition to psychosis, piquindone has also been found to be effective in the treatment of Tourette's syndrome in numerous clinical studies.[8][9][10][11]
References
- Cohen JD, Van Putten T, Marder S, Berger PA, Stahl SM (October 1987). "The efficacy of piquindone, a new atypical neuroleptic, in the treatment of the positive and negative symptoms of schizophrenia". Journal of Clinical Psychopharmacology. 7 (5): 324–9. doi:10.1097/00004714-198710000-00006. PMID 2890671.
- Olson GL, Cheung HC, Morgan KD, et al. (September 1981). "A dopamine receptor model and its application in the design of a new class of rigid pyrrolo[2,3-g]isoquinoline antipsychotics". Journal of Medicinal Chemistry. 24 (9): 1026–34. doi:10.1021/jm00141a002. PMID 6116805.
- Davidson AB, Boff E, MacNeil DA, Wenger J, Cook L (1983). "Pharmacological effects of Ro 22-1319: a new antipsychotic agent". Psychopharmacology. 79 (1): 32–9. doi:10.1007/BF00433013. PMID 6132425.
- Nakajima T, Iwata K (November 1984). "[3H]Ro 22-1319 (piquindone) binds to the D2 dopaminergic receptor subtype in a sodium-dependent manner". Molecular Pharmacology. 26 (3): 430–8. PMID 6149457.
- Pugh MT, O'Boyle KM, Molloy AG, Waddington JL (1985). "Effects of the putative D-1 antagonist SCH 23390 on stereotyped behaviour induced by the D-2 agonist RU24213". Psychopharmacology. 87 (3): 308–12. doi:10.1007/BF00432713. PMID 2934758.
- Molloy AG, O'Boyle KM, Pugh MT, Waddington JL (July 1986). "Locomotor behaviors in response to new selective D-1 and D-2 dopamine receptor agonists, and the influence of selective antagonists". Pharmacology Biochemistry and Behavior. 25 (1): 249–53. doi:10.1016/0091-3057(86)90262-5. PMID 3529126.
- Collin S, Vercauteren DP, Vanderveken D, Evrard G, Durant F (March 1989). "Structural requirements of Na+-dependent antidopaminergic agents: Tropapride, Piquindone, Zetidoline, and Metoclopramide. Comparison with Na+-independent ligands". Journal of Computer-aided Molecular Design. 3 (1): 39–53. doi:10.1007/BF01590994. PMID 2715795.
- Uhr SB, Berger PA, Pruitt B, Stahl SM (October 1984). "Treatment of Tourette's syndrome with RO22-1319, a D-2-receptor antagonist". The New England Journal of Medicine. 311 (15): 989. doi:10.1056/NEJM198410113111517. PMID 6147753.
- Uhr SB, Pruitt B, Berger PA, Stahl SM (April 1986). "Case report of four patients with Tourette syndrome treated with piquindone, a D2 receptor antagonist". Journal of Clinical Psychopharmacology. 6 (2): 128–30. doi:10.1097/00004714-198604000-00028. PMID 2871057.
- Uhr SB, Pruitt B, Berger PA, Stahl SM (July 1986). "Improvement of symptoms in Tourette syndrome by piquindone, a novel dopamine-2 receptor antagonist". International Clinical Psychopharmacology. 1 (3): 216–20. doi:10.1097/00004850-198607000-00004. PMID 3549873.
- Jiménez-Jiménez FJ, García-Ruiz PJ (2001). "Pharmacological options for the treatment of Tourette's disorder". Drugs. 61 (15): 2207–20. doi:10.2165/00003495-200161150-00005. PMID 11772131.