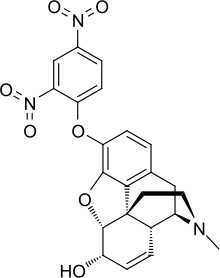
2,4-Dinitrophenylmorphine
2,4-Dinitrophenylmorphine is an analog of morphine in which a hydroxyl group is substituted with a dinitro phenoxy group.[1][2]
 | |
| Names | |
|---|---|
| IUPAC name
(1S,5R,13R,14S,17R)-10-(2,4-dinitrophenoxy)-4-methyl-12-oxa-4-azapentacyclo [9.6.1.01,13.05,17.07,18]octadeca-7(18),8,10,15-tetraen-14-ol | |
| Other names
7,8-Didehydro-3-α-(2',4'- dinitro)phenoxy-4,5-α-epoxy-17-methylmorphinan-6-α-ol | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H21N3O7 | |
| Molar mass | 451.43 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
Being an analog of morphine, it would be expected to have the same effects on the body as a typical opioid. Also, as dinitrophenol is a metabolic and respiratory stimulant, this morphine derivative was invented in Austria in 1931 as a narcotic analgesic with less potential to depress respiration.[3]
References
- Eddy, N. B.; Sumwalt, M. (1939). "Studies of Morphine, Codeine, and Their Derivatives. XV. 2,4-Dinitrophenylmorphine" (PDF). Journal of Pharmacology and Experimental Therapeutics. 67 (2): 127–141.
- Huggins, R. A.; Bryan, A. R. (1951). "Some cardiovascular actions of dinitrophenylmorphine hydrochloride". Texas Reports on Biology and Medicine. 9 (2): 314–318. PMID 14835466.
- Reynolds and Randall, "Morphine and Allied Drugs", U of Toronto, 1957 pp 203 et al.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.