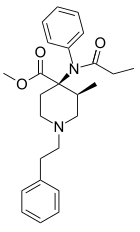
Lofentanil
Lofentanil is one of the most potent opioid analgesics known and is an analogue of fentanyl, which was developed in 1960. It is most similar to the highly potent opioid carfentanil (4-carbomethoxyfentanyl), only slightly more potent. Lofentanil can be described as 3-methylcarfentanil, or 3-methyl-4-carbomethoxyfentanyl. While 3-methylfentanyl is considerably more potent than fentanyl itself, lofentanil is only slightly stronger than carfentanil.[1][2] This suggests that substitution at both the 3 and 4 positions of the piperidine ring introduces steric hindrance which prevents μ-opioid affinity from increasing much further. As with other 3-substituted fentanyl derivatives such as ohmefentanyl, the stereoisomerism of lofentanil is very important, with some stereoisomers being much more potent than others.
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Lofentanil; methyl (3S,4R)-1-(2-cyclohexylethyl)-4 -(cyclohexyl-propanoylamino)-3-methylpiperidine-4-carboxylate |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H32N2O3 |
| Molar mass | 408.542 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Lofentanil is very similar to carfentanil in effects, but has a longer duration of action.[3] This makes it unsuitable for most practical applications, with carfentanil being the preferred agent for tranquilizing large animals, and short-acting derivatives such as sufentanil or remifentanil being preferred for medical use in human surgical procedures. The long duration and high lipophilicity of lofentanil has been suggested as an advantage for certain types of analgesia,[4] but the main application for lofentanil at the present time is research into opioid receptors.[5][6]
Side effects of lofentanyl analogs are similar to those of fentanyl itself, which include itching, nausea, and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[7] Side effects from lofentanil might be particularly problematic given its reportedly long duration of action. Another side effect which is characteristic of fentanyl and its derivatives is their tendency to rapidly induce tolerance, due to their high binding affinity triggering rapid internalization of chronically activated opiate receptors.[8] This might be expected to be a particular problem with lofentanil as it is not only one of the most potent drugs in the series, but also is longer acting than most other fentanyl analogues, meaning that development of tolerance triggered by receptor over-activation could be rapid.
In addition to acting on the μ-opioid receptor, lofentanil has also been found to act as a full agonist of the κ-opioid receptor (Ki = 8.2 nM; EC50 = 153 nM; Emax = 100%).[9]
- Ohmecarfentanil (RTI-4614-38) is 30,000 times more potent than morphine in the rhesus monkey single dose suppression test.[10] This makes it the most potent opioid known at this time even surpassing lofentanil. Another name for this agent is Ohlofentanil.
References
- Gommeren W, Leysen JE (Jul 1982). "Binding properties of 3H-lofentanil at the opiate receptor". Archives Internationales de Pharmacodynamie et de Thérapie. 258 (1): 171–3. PMID 6291471.
- Meert TF, Lu HR, van Craenndonck H, Janssen PA (September 1988). "Comparison between epidural fentanyl, sufentanil, carfentanil, lofentanil and alfentanil in the rat: analgesia and other in vivo effects". European Journal of Anaesthesiology. 5 (5): 313–21. PMID 2905988.
- Laduron PM, Janssen PF (August 1982). "Axoplasmic transport and possible recycling of opiate receptors labelled with 3H-lofentanil". Life Sciences. 31 (5): 457–62. doi:10.1016/0024-3205(82)90331-9. PMID 6182434.
- Foldes FF (1991). "Pain control with intrathecally and peridurally administered opioids and other drugs". Anaesthesiologie und Reanimation. 16 (5): 287–98. PMID 1683773.
- Maguire P, Tsai N, Kamal J, Cometta-Morini C, Upton C, Loew G (March 1992). "Pharmacological profiles of fentanyl analogs at mu, delta and kappa opiate receptors". European Journal of Pharmacology. 213 (2): 219–25. doi:10.1016/0014-2999(92)90685-w. PMID 1355735.
- Huang XQ, Jiang HL, Luo XM, Chen KX, Zhu YC, Ji RY, Cao Y (June 2000). "Study on mechanism of interaction of nociceptin and opioids binding with opioid receptor-like 1 receptor". Acta Pharmacologica Sinica. 21 (6): 536–46. PMID 11360688.
- Mounteney J, Giraudon I, Denissov G, Griffiths P (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". The International Journal on Drug Policy. 26 (7): 626–31. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
- Bot G, Blake AD, Li S, Reisine T (June 1998). "Fentanyl and its analogs desensitize the cloned mu opioid receptor". The Journal of Pharmacology and Experimental Therapeutics. 285 (3): 1207–18. PMID 9618424.
- Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J (January 2006). "Pharmacological profiles of opioid ligands at kappa opioid receptors". BMC Pharmacology. 6 (1): 3. doi:10.1186/1471-2210-6-3. PMC 1403760. PMID 16433932.
- Carroll, F. Ivy; Lewin, Anita H.; Mascarella, S. Wayne; Seltzman, Herbert H.; Reddy, P. Anantha (2020). "Designer drugs: a medicinal chemistry perspective (II)". Annals of the New York Academy of Sciences. doi:10.1111/nyas.14349.