Biphalin
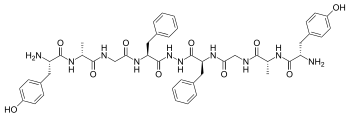
Biphalin is a dimeric enkephalin endogenous peptide (Tyr-D-Ala-Gly-Phe-NH)2 composed of two tetrapeptides derived from enkephalins, connected 'tail-to-tail' by a hydrazide bridge.[1] The presence of two distinct pharmacophores confers on biphalin a high affinity for both μ and δ opioid receptors (with an EC50 of about 1-5 nM for both μ and δ receptors), therefore it has analgesic activity.[2] Biphalin presents a considerable antinociceptive profile. In fact, when administered intracerebroventricularly in mice, biphalin displays a potency almost 7-fold greater than that of the ultra-potent alkaloid agonist, etorphine and 7000-fold greater than morphine; biphalin and morphine were found to be equipotent after intraperitoneal administration. The extraordinary in vivo potency shown by this compound is coupled with low side-effects, in particular, to produce no dependency in chronic use.[3] For these reasons, several efforts have been carried out in order to obtain more information about structure-activity relationship (SAR). Results clearly indicate that, at least for μ receptor binding, the presence of two pharmacophores is not necessary;[2] Tyr1 is indispensable for analgesic activity, while replacing Phe at the position 4 and 4' with non-aromatic, but lipophilic amino acids does not greatly change the binding properties[2] and in general 4,4' positions are found to be important to design biphalin analogues with increased potency and modified μ/δ selectivity.[4][5] The hydrazide linker is not fundamental for activity or binding, and it can be conveniently substituted by different conformationally constrained cycloaliphatic diamine linkers.[6]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C46H56N10O10 |
| Molar mass | 909.014 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Flippen-Anderson JL, Deschamps JR, George C, Hruby VJ, Misicka A, Lipkowski AW (March 2002). "Crystal structure of biphalin sulfate: a multireceptor opioid peptide". The Journal of Peptide Research. 59 (3): 123–33. doi:10.1034/j.1399-3011.2002.01967.x. PMID 11985706.
- Lipkowski AW, Misicka A, Davis P, Stropova D, Janders J, Lachwa M, et al. (September 1999). "Biological activity of fragments and analogues of the potent dimeric opioid peptide, biphalin". Bioorganic & Medicinal Chemistry Letters. 9 (18): 2763–6. doi:10.1016/S0960-894X(99)00464-3. PMID 10509931.
- Horan PJ, Mattia A, Bilsky EJ, Weber S, Davis TP, Yamamura HI, et al. (June 1993). "Antinociceptive profile of biphalin, a dimeric enkephalin analog". The Journal of Pharmacology and Experimental Therapeutics. 265 (3): 1446–54. PMID 8389867.
- Li G, Haq W, Xiang L, Lou BS, Hughes R, De Leon IA, et al. (March 1998). "Modifications of the 4,4'-residues and SAR studies of Biphalin, a highly potent opioid receptor active peptide". Bioorganic & Medicinal Chemistry Letters. 8 (5): 555–60. doi:10.1016/S0960-894X(98)00065-1. PMID 9871617.
- Mollica A, Pinnen F, Feliciani F, Stefanucci A, Lucente G, Davis P, et al. (May 2011). "New potent biphalin analogues containing p-fluoro-L-phenylalanine at the 4,4' positions and non-hydrazine linkers". Amino Acids. 40 (5): 1503–11. doi:10.1007/s00726-010-0760-7. PMC 5689474. PMID 20924622.
- Mollica A, Davis P, Ma SW, Lai J, Porreca F, Hruby VJ (May 2005). "Synthesis and biological evaluation of new biphalin analogues with non-hydrazine linkers". Bioorganic & Medicinal Chemistry Letters. 15 (10): 2471–5. doi:10.1016/j.bmcl.2005.03.067. PMID 15863299.