7-Hydroxymitragynine
7-Hydroxymitragynine is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as Kratom.[2] It is often referred to as ‘7-OH’. It was first described in 1994[3] and is a natural product derived from the mitragynine present in the Kratom leaf. It is considered an oxidized derivative of mitragynine.[4]
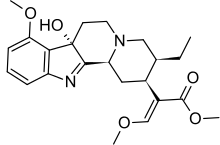 | |
| Names | |
|---|---|
| IUPAC name
Methyl (E)-2-[(2S,3S,7aS,12bS)-3-ethyl-7a-hydroxy-8-methoxy-2,3,4,6,7,12b-hexahydro-1H-indolo[2,3-a]quinolizin-2-yl]-3-methoxyprop-2-enoate | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H30N2O5 | |
| Molar mass | 414.502 g·mol−1 |
| log P | 1.266 |
| Acidity (pKa) | 12.203 |
| Basicity (pKb) | 1.794 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Metabolism
After a kratom study, it was revealed that 7-OH converts into Mitragynine pseudoindoxyl.[5]

| Compound | Affinities (Ki) | Ratio | Ref | ||
|---|---|---|---|---|---|
| MOR | DOR | KOR | MOR:DOR:KOR | ||
| 7-Hydroxymitragynine | 13.5 | 155 | 123 | 1:11:9 | [6] |
| Mitragynine | 7.24 | 60.3 | 1,100 | 1:8:152 | [6] |
| Mitragynine pseudoindoxyl | 0.087 | 3.02 | 79.4 | 1:35:913 | [6] |
Other analogs from 7-OH
Neuropathic Pain
(E)-Methyl 2-((2S,3S,7aS,12bS)-3-ethyl-7a-hydroxy-8-methoxy-1,2,3,4,6,7,7a,12b-octahydroindolo[2,3-a]quinolizin-2-yl)-3-methoxyacrylate (7-hydroxymitragynine), a main active constituent of the traditional herbal medicine Mitragyna speciosa, is an indole alkaloid that is structurally different from morphine. 7-Hydroxymitragynine induces a potent antinociceptive effect on mouse acute pain through μ-opioid receptors. In this study, we developed dual-acting μ- and δ-opioid agonists MGM-15 and MGM-16 from 7-hydroxymitragynine for the treatment of acute and chronic pain. MGM-16 showed a higher potency than that of 7-hydroxymitragynine and MGM-15 in in vitro and in vivo assays. MGM-16 exhibited a high affinity for μ- and δ-opioid receptors, with K(i) values of 2.1 and 7.0 nM, respectively. MGM-16 showed μ- and δ-opioid full agonistic effects in a guanosine 5'-O-(3-[(35)S]thiotriphosphate) binding assay and in a functional test using electrically elicited guinea pig ileum and mouse vas deferens contractions. Systemic administration of MGM-16 produced antinociceptive effects in a mouse acute pain model and antiallodynic effects in a chronic pain model. The antinociceptive effect of MGM-16 was approximately 240 times more potent than that of morphine in a mouse tail-flick test, and its antiallodynic effect was approximately 100 times more potent than that of gabapentin in partial sciatic nerve-ligated mice, especially with oral administration. The antinociceptive effect of MGM-16 was completely and partially blocked by the μ-selective antagonist β-funaltrexamine hydrochloride (β-FNA) and by the δ-selective antagonist naltrindole, respectively, in a tail-flick test. The antiallodynic effect of MGM-16 was completely blocked by β-FNA and naltrindole in a neuropathic pain model. These findings suggest that MGM-16 could become a class of a compound with potential therapeutic utility for treating neuropathic pain.[7]
See also
- Ajmalicine
- Mitragynine
- Mitragynine pseudoindoxyl
- Mitraphylline
- β-Prodine - molecule which overlays with 7-hydroxymitragynine's opioid QSAR
References
- Chemical Abstracts Service: Columbus, OH, 2004; RN 174418-82-7 (accessed via SciFinder Scholar, version 2007.3; November 30, 2011)
- Matsumoto K, Horie S, Ishikawa H, Takayama H, Aimi N, Ponglux D, Watanabe K (March 2004). "Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa". Life Sciences. 74 (17): 2143–55. doi:10.1016/j.lfs.2003.09.054. PMID 14969718.
- Ponglux, Dhavadee; Wongseripipatana, Sumphan; Takayama, Hiromitsu; Kikuchi, Masae; Kurihara, Mika; Kitajima, Mariko; Aimi, Norio; Sakai, Shin-ichiro (1994). "A New Indole Alkaloid, 7 α-Hydroxy-7H-mitragynine, from Mitragyna speciosa in Thailand". Planta Medica. 60 (6): 580–581. doi:10.1055/s-2006-959578. ISSN 0032-0943. PMID 17236085.
- "7-Hydroxymitragynine - Green Leaf Kratom - Kratom Blogs Archives". Green Leaf Kratom. 2020-08-19. Retrieved 2020-08-22.
- Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A, Pagirsky J, Eans SO, Medina JM, Xu J, Pan YX, Borics A, Pasternak GW, McLaughlin JP, Majumdar S (2016). "Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit β-Arrestin-2". J. Med. Chem. 59 (18): 8381–97. doi:10.1021/acs.jmedchem.6b00748. PMC 5344672. PMID 27556704.
- Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, Watanabe K, Murayama T, Horie S (2002). "Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands". J. Med. Chem. 45 (9): 1949–56. doi:10.1021/jm010576e. PMID 11960505.
- https://pubmed.ncbi.nlm.nih.gov/24345467/
External links
- Takayama H (August 2004). "Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa" (pdf). Chemical & Pharmaceutical Bulletin. 52 (8): 916–28. doi:10.1248/cpb.52.916. PMID 15304982. - synthesis of 7-hydroxymitragynine from mitragynine