Gay-Lussac's law
Gay's law (more correctly referred to as Amontons's law) states that the pressure of a given mass of gas varies directly with the absolute temperature of the gas, when the volume is kept constant.[1]
| Part of a series on |
| Continuum mechanics |
|---|
Mathematically, it can be written as: . It is a special case of the ideal gas law.
Gay-Lussac is incorrectly recognized for the Pressure Law which established that the pressure of an enclosed gas is directly proportional to its temperature and which he was the first to formulate (c. 1809).[2] He is also sometimes credited[3][4][5] with being the first to publish convincing evidence that shows the relationship between the pressure and temperature of a fixed mass of gas kept at a constant volume.[4]
These laws are also known variously as the Pressure Law or Amontons's law and Dalton's law respectively.[3][4][5][6]
Law of combining volumes
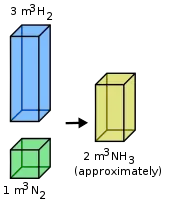
The law of combining volumes states that, when gases react together they do so in volume which bears simple whole number ratio provided that the temperature and pressure of the reacting gases and their products remain constant
The ratio between the volumes of the reactant gases and the gaseous products can be expressed in simple whole numbers.
For example, Gay-Lussac found that two volumes of hydrogen and one volume of oxygen would react to form two volumes of gaseous water. Based on Gay-Lussac's results, Amedeo Avogadro hypothesized that, at the same temperature and pressure, equal volumes of gas contain equal numbers of molecules (Avogadro's law). This hypothesis meant that the previously stated result
- 2 volumes of hydrogen + 1 volume of oxygen = 2 volume of gaseous water
could also be expressed as
- 2 molecules of hydrogen + 1 molecule of oxygen = 2 molecule of water.
It can also be expressed in another way of example, 100 mL of hydrogen combine with 50 mL of oxygen to give 100 mL of water vapour. Hydrogen(100 mL) + Oxygen(50 mL) = Water(100 mL)
Thus, the volumes of hydrogen and oxygen which combine ( i.e., 100mL and 50mL ) bear a simple ratio of 2:1.
The law of combining gases was made public by Joseph Louis Gay-Lussac in 1808.[7][8] Avogadro's hypothesis, however, was not initially accepted by chemists until the Italian chemist Stanislao Cannizzaro was able to convince the First International Chemical Congress in 1860.[9]
Pressure-temperature law
This law is often referred to as Gay-Lussac's law of pressure–temperature, between 1800 and 1802, discovered the relationship between the pressure and temperature of a fixed mass of gas kept at a constant volume.[10][11][12] Gay Lussac discovered this while building an "air thermometer".
The pressure of a gas of fixed mass and fixed volume is directly proportional to the gas's absolute temperature.
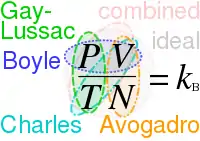
If a gas's temperature increases, then so does its pressure if the mass and volume of the gas are held constant. The law has a particularly simple mathematical form if the temperature is measured on an absolute scale, such as in kelvins. The law can then be expressed mathematically as
or
where:
- P is the pressure of the gas,
- T is the temperature of the gas (measured in kelvins),
- k is a constant.
This law holds true because temperature is a measure of the average kinetic energy of a substance; as the kinetic energy of a gas increases, its particles collide with the container walls more rapidly, thereby exerting increased pressure.
For comparing the same substance under two different sets of conditions, the law can be written as:
Because Amontons discovered the law beforehand, Gay-Lussac's name is now generally associated within chemistry with the law of combining volumes discussed in the section above. Some introductory physics textbooks still define the pressure-temperature relationship as Gay-Lussac's law.[13][14][15] Gay-Lussac primarily investigated the relationship between volume and temperature and published it in 1802, but his work did cover some comparison between pressure and temperature.[16] Given the relative technology available to both men, Amontons was only able to work with air as a gas, where Gay-Lussac was able to experiment with multiple types of common gases, such as oxygen, nitrogen, and hydrogen.[17] Gay-Lussac did attribute his findings to Jacques Charles because he used much of Charles's unpublished data from 1787 – hence, the law became known as Charles's law or the Law of Charles and Gay-Lussac.[18]
Gay-Lussac's (Amontons') law, Charles's law, and Boyle's law form the combined gas law. These three gas laws in combination with Avogadro's law can be generalized by the ideal gas law.
Expansion of gases
Gay-Lussac used the formula acquired from ΔV/V = αΔT to define the rate of expansion α for gases. For air he found a relative expansion ΔV/V = 37.50% and obtained a value of α = 37.50%/100°C = 1/266.66°C which indicated that the value of absolute zero was approximately 266.66°C below 0°C.[19] The value of the rate of expansion α is approximately the same for all gases and this is also sometimes referred to as Gay-Lussac's Law.
See also
- Avogadro's law – Relationship between volume and number of moles of a gas at constant temperature and pressure.
- Boyle's law – Relationship between pressure and volume in a gas at constant temperature
- Charles's law – Relationship between volume and temperature of a gas at constant pressure
- Combined gas law – Combination of Charles', Boyle's and Gay-Lussac's gas laws
References
- "Gay-Lussac's Law". LibreTexts. 2016-06-27. Retrieved 5 December 2018.
- Lagassé, Paul (2016), "Joseph Louis Gay-Lussac", Columbia Electronic Encyclopedia (6th Edition, Q2 ed.), Columbia University, ISBN 978-0787650155
- Palmer, WP (1991), "Philately, Science Teaching and the History of Science" (PDF), Lab Talk, 35 (1): 30–31
- Holbrow, CH; Amato, JC (2011), "What Gay-Lussac didn't tell us", Am. J. Phys., 79 (1): 17, Bibcode:2011AmJPh..79...17H, doi:10.1119/1.3485034
- Spurgin, CB (1987), "Gay-Lussac's gas-expansivity experiments and the traditional mis-teaching of 'Charles's Law'", Annals of Science, 44 (5): 489–505, doi:10.1080/00033798700200321
- Crosland MP (1961), "The Origins of Gay-Lussac's Law of Combining Volumes of Gases", Annals of Science, 17 (1): 1, doi:10.1080/00033796100202521
- Gay-Lussac (1809) "Mémoire sur la combinaison des substances gazeuses, les unes avec les autres" (Memoir on the combination of gaseous substances with each other), Mémoires de la Société d'Arcueil 2: 207–234. Available in English at: Le Moyne College.
- "Joseph-Louis Gay-Lussac". chemistryexplained.com.
- Hartley Harold (1966). "Stanislao Cannizzaro, F.R.S. (1826–1910) and the First International Chemical Conference at Karlsruhe". Notes and Records of the Royal Society of London. 21 (1): 56–63. doi:10.1098/rsnr.1966.0006. S2CID 58453894.
- Barnett, Martin K. (Aug 1941), "A brief history of thermometry", Journal of Chemical Education, 18 (8): 358, Bibcode:1941JChEd..18..358B, doi:10.1021/ed018p358. Extract.
- "Thall's History of Gas Laws". Archived from the original on 2010-09-08. Retrieved 2010-07-16.
- See:
- Amontons, G. (presented 1699, published 1732) "Moyens de substituer commodément l'action du feu à la force des hommes et des chevaux pour mouvoir les machines" (Ways to conveniently substitute the action of fire for the force of men and horses in order to power machines), Mémoires de l’Académie des sciences de Paris, 112–126; see especially pages 113–117.
- Amontons, G. (presented 1702, published 1743) "Discours sur quelques propriétés de l'Air, & le moyen d'en connoître la température dans tous les climats de la Terre" (Discourse on some properties of air and on the means of knowing the temperature in all climates of the Earth), Mémoires de l’Académie des sciences de Paris, 155–174.
- See also: Fontenelle, B. B. (1743) "Sur une nouvelle proprieté de l'air, et une nouvelle construction de Thermométre" (On a new property of the air and a new construction of thermometer), Histoire de l'Academie royale des sciences, 1–8.
- Tippens, Paul E. (2007). Physics, 7th ed. McGraw-Hill. 386–387.
- Cooper, Crystal (Feb. 11, 2010). "Gay-Lussac's Law". Bright Hub Engineering. Retrieved from http://www.brighthubengineering.com/hvac/26213-gay-lussacs-law/ on July 8, 2013.
- Verma, K.S. - Cengage Physical Chemistry Part 1 - Section 5.6.3
- Crosland, Maurice P. (2004). Gay-Lussac: Scientist and Bourgeois. Cambridge University Press. 119–120.
- Asimov, Isaac (1966). Understanding Physics – Motion, Sound, and Heat. Walker and Co. 191–192.
- Gay-Lussac (1802), "Recherches sur la dilatation des gaz et des vapeurs" (Researches on the expansion of gases and vapors), Annales de Chimie 43: 137–175. On page 157, Gay-Lussac mentions the unpublished findings of Charles: "Avant d'aller plus loin, je dois prévenir que quoique j'eusse reconnu un grand nombre de fois que les gaz oxigène, azote, hydrogène et acide carbonique, et l'air atmosphérique se dilatent également depuis 0° jusqu'a 80°, le cit. Charles avait remarqué depuis 15 ans la même propriété dans ces gaz ; mais n'avant jamais publié ses résultats, c'est par le plus grand hasard que je les ai connus." (Before going further, I should inform [you] that although I had recognized many times that the gases oxygen, nitrogen, hydrogen, and carbonic acid [i.e., carbon dioxide], and atmospheric air also expand from 0° to 80°, citizen Charles had noticed 15 years ago the same property in these gases; but having never published his results, it is by the merest chance that I knew of them.) Available in English at: Le Moyne College.
- Gay-Lussac (1802). "Recherches sur la dilatation des gaz et des vapeurs". Annales de chimie, ou, Recueil de mémoires concernant la chimie (in French).
Further reading
- Castka, Joseph F.; Metcalfe, H. Clark; Davis, Raymond E.; Williams, John E. (2002). Modern Chemistry. Holt, Rinehart and Winston. ISBN 978-0-03-056537-3.
- Guch, Ian (2003). The Complete Idiot's Guide to Chemistry. Alpha, Penguin Group Inc. ISBN 978-1-59257-101-7.
- Mascetta, Joseph A. (1998). How to Prepare for the SAT II Chemistry. Barron's. ISBN 978-0-7641-0331-5.