Hydrastine
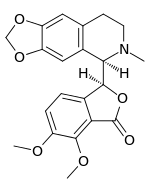
Hydrastine is an isoquinoline alkaloid which was discovered in 1851 by Alfred P. Durand.[1] Hydrolysis of hydrastine yields hydrastinine, which was patented by Bayer as a haemostatic drug[2] during the 1910s. It is present in Hydrastis canadensis (thus the name) and other plants of the family Ranunculaceae.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.849 |
| Chemical and physical data | |
| Formula | C21H21NO6 |
| Molar mass | 383.400 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Total synthesis
The first attempt for the total synthesis of hydrastine was reported by Sir Robert Robinson and co-workers[3] in 1931. Following studies[4][5] where the synthesis of the key lactonic amide intermediate (structure 4 in figure) was the most troublesome, the major breakthrough was achieved in 1981 when J. R. Falck and co-workers[6] reported a four-step total synthesis of hydrastine from simple starting materials. The key step in the Flack synthesis was using a Passerini reaction to construct the lactonic amide intermediate 4.
Starting from a simple phenylbromide variant 1, alkylation reaction with lithium methylisocyanide gives the isocyanide intermediate 2. Reacting isocyanide intermediate 2 with opianic acid 3 initiated the intramolecular Passerini reaction to give the key lactonic amide intermediate 4. The tetrahydro-isoquinolin ring was formed by first a ring-closure reaction under dehydration conditions using POCl3 and then a catalyzed hydrogenation using PtO2 as the catalyst. Finally, hydrastine was synthesized by installing the N-methyl group via reductive amination reaction with formaldehyde.
See also
- Bicuculline (very similar in structure)
References
- Perrins JD (July 1862). "On Hydrastine, an Alkaloid Occurring in Hydrastis Canadensis". Pharmaceutical Journal: A Weekly Record of Pharmacy and Allied Sciences. J. Churchill: 547–.CS1 maint: date and year (link)
- Römpp CD, Georg Thieme Verlag, 2006
- Hope E, Pyman FL, Remfry FG, Robinson R (1931). "XXXI.—A synthesis of hydrastine. Part I". J. Chem. Soc. 0 (0): 236–247. doi:10.1039/JR9310000236. ISSN 0368-1769.
- Haworth RD, Pinder AR, Robinson R (1950). "Synthesis of Hydrastine". Nature. 165 (4196): 529–529. doi:10.1038/165529a0. ISSN 0028-0836.
- Haworth RD, Pinder AR (1950). "360. A new route to the phthalide-isoquinoline bases, and a synthesis of (–)-hydrastine". J. Chem. Soc. 0 (0): 1776–1780. doi:10.1039/JR9500001776. ISSN 0368-1769.
- Falck JR, Manna S (1981). "An intramolecular passerini reaction: Synthesis of hydrastine". Tetrahedron Letters. 22 (7): 619–620. doi:10.1016/S0040-4039(01)92504-3. ISSN 0040-4039.
External links
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 14 (11th ed.). Cambridge University Press. p. 34.
