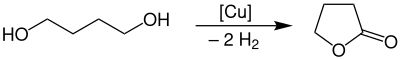gamma-Butyrolactone
γ-Butyrolactone (GBL) is a hygroscopic colorless, water-miscible liquid with a weak characteristic odor. It is the simplest 4-carbon lactone. It is mainly used as an intermediate in the production of other chemicals, e.g. methyl-2-pyrrolidone.[4] In humans GBL acts as a prodrug for γ-hydroxybutyric acid (GHB), and it is used as a recreational CNS depressant with effects similar to barbiturates.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Oxolan-2-one | |
| Other names
Dihydrofuran-2(3H)-one GBL Butyrolactone 1,4-Lactone 4-Butyrolactone 4-Hydroxybutyric acid lactone gamma-Hydroxybutyric acid lactone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.282 |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6O2 | |
| Molar mass | 86.090 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.1286 g/mL (15 °C), 1.1296 g/mL (20 °C) |
| Melting point | −43.53 °C (−46.35 °F; 229.62 K) |
| Boiling point | 204 °C (399 °F; 477 K) |
| Miscible | |
| Solubility | Soluble in CCl4, methanol, ethanol, acetone, benzene, ethyl ether |
| log P | −0.76[3] |
| Acidity (pKa) | 4.5 |
Refractive index (nD) |
1.435, 1.4341 (20 °C) |
| Viscosity | 1.7 cP (25 °C) |
| Hazards | |
| Main hazards | Toxic and flammable |
| H318, H302, H336 | |
| P264, P270, P280, P301+312, P305+351+338, P403+233, P501 | |
| Flash point | 98 °C (208 °F; 371 K) (closed cup) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
17.2 mL/kg (orally, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Occurrence
GBL has been found in extracts from samples of unadulterated wines.[5][6] This finding indicates that GBL is a naturally occurring component in some wines and may be present in similar products. The concentration detected was approximately 5 μg/mL and was easily observed using a simple extraction technique followed by GC/MS analysis. GBL can be found in cheese flavourings but typically results in a content of 0.0002% GBL in the final foodstuff.[7]
Production and synthesis
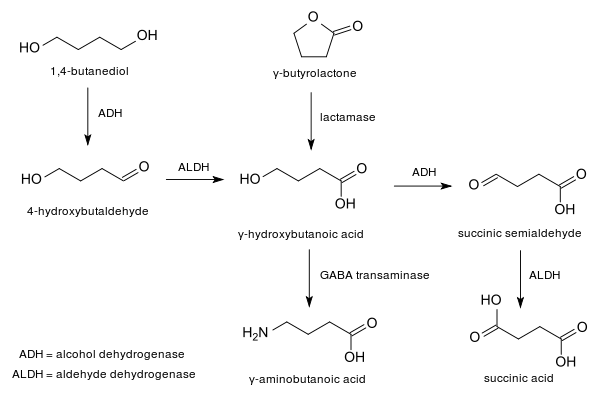
γ-Butyrolactone is produced industrially by dehydrogenation of 1,4-butanediol at a temperature of 180–300 °C and atmospheric pressure in the presence of a copper catalyst.[4]
The yield of this process is approximately 95%. The purification takes place with a liquid-gas-phase extraction.[4]
In the laboratory, it may also be obtained via the oxidation of tetrahydrofuran (THF), for example with aqueous sodium bromate.[8] An alternative route proceeds from GABA using the Sandmeyer reaction.[9]
Reactions
As a lactone, GBL is hydrolyzed under basic conditions, for example in a sodium hydroxide solution into sodium gamma-hydroxybutyrate, the sodium salt of gamma-hydroxybutyric acid. In acidic water, a mixture of the lactone and acid forms coexist in an equilibrium. These compounds then may go on to form the polymer poly(4-hydroxybutyrate) as well as the dimer 1,6-Dioxecane-2,7-dione. When treated with a non-nucleophilic base, such as lithium diisopropylamide, GBL undergoes deprotonation alpha to the carbonyl. The related compound caprolactone can be used to make a polyester in this manner.
Polymerization
The ring-opening polymerization of butyrolactone gives polybutyrolactone. The resulting reverts to the monomer by thermal cracking.[10][11] It is claimed that poly(GBL) is competitive with commercial biomaterial poly(4-hydroxybutyrate), or P4HB. It is further claimed that poly(GBL) is cheaper to make than P4HB, although both are bio-derived.[10][12]
Uses
Butyrolactone is principally a precursor to other chemicals. Reaction with methylamine gives NMP. Ammonia gives pyrrolidone. It is also used as a solvent in lotions and some polymers.[4]
Butyrolactone, with its wide liquid range, chemical stability and high dielectric constant, is used in some batteries but primarily is found in electrolytic capacitors as the organic solvent. In this capacity, it is frequently mixed with a small ratio of ethylene glycol, "9:1" being common, to vary internal resistivity.[13]
Recently GBL has become an important solvent for the development of organic perovskites (such as Methylammonium lead halide), novel solar cell materials that can be processed from solution. GBL is a good solvent for the precursor salts such as lead halides and methylammonium iodide or formamidinium iodide. Often mixtures of GBL and DMSO yield best results.[14] GBL is an alternative to the more toxic solvent dimethylformamide that was used in the earlier stages of the development of the perovskite solar cell.
Pharmacology
GBL is not active in its own right; its mechanism of action stems from its identity as a prodrug of GHB.
Pharmacokinetics
GBL is rapidly converted into GHB by paraoxonase (lactonase) enzymes, found in the blood.[15][16] Animals which lack these enzymes exhibit no effect from GBL.[15] GBL is more lipophilic (fat soluble) than GHB, and so is absorbed faster and has higher bioavailability. Because of these pharmacokinetic differences, GBL tends to be more potent and faster-acting than GHB, but has a shorter duration; whereas the related compound 1,4-butanediol (1,4-B) tends to be slightly less potent, slower to take effect but longer-acting than GHB.[17]
Nutritional supplement
Due to its property of being a prodrug of GHB which increases sleep related growth hormone (GH) secretion,[18] GBL was sold as a nutritional supplement after the scheduling of GHB, under the names Revivarant and Renewtrient[19] until they were banned by the FDA.
Recreational drug
GBL is a prodrug of GHB and its recreational use comes entirely as a result of this.[20] GBL overdose can cause irrational behaviour, severe sickness, coma and death.[21]
To bypass GHB restriction laws, home synthesis kits were introduced to transform GBL and/or 1,4-B into GHB.


GBL has a distinctive taste and odour, described as being comparable to stale water, synthetic melon aroma or burnt plastic. This differs significantly from GHB, which is described as having a decidedly "salty" taste.[22]
Due to the fact that those with limited chemistry knowledge can make GBL with easy to get precursors, it has become quite popular among young people in French nightclubs.[23][24]
Dangers
If taken undiluted by mouth, GBL can cause esophageal and gastro-intestinal irritation. It is possible for oral ingestion of GBL to cause nausea and other similar problems, possibly more so than with GHB.
GHB has biphasic effects, a euphoric effect at low doses (the reason for the term liquid ecstasy), and a sedative effect[25] at higher doses. As a result of this sedation it can cause unconsciousness.[26] When combined with alcohol the increased sedation and risk of vomiting results in a high risk of fatality. Many harm reduction organisations suggest never mixing the two drugs as a result.[27][28]
There have been news reports of several deaths associated with GBL, usually in combination with alcohol or other depressants.[29]
Addictiveness and dependence
Frequent use of GHB/GBL, even when taken long-term and in moderate doses, does not appear to cause significant physical dependency in the majority of its users. In many people, quitting or temporarily abstaining from use of the drugs is achieved with minimal or no difficulty. However, when consumed in excessive amounts with a high frequency of dosing, physical and psychological dependence can develop.[30] Management of GBL dependence involves considering the person's age, comorbidity and the pharmacological pathways of GBL.[31]
GHB/GBL users can adopt a '24/7' dosing regime.[32] This is where the user has become tolerant to the effects of the drug, increasing the dosage and frequency of dosage simply to avoid withdrawal symptoms.
For those users who do report withdrawal symptoms upon quitting the use of GHB/GBL, symptoms seem to depend on the dosage and the length of time the drug was used. Light to moderate users often experience insomnia and sleep-related problems, whereas heavy, prolonged use can cause severe withdrawal symptoms similar to Benzodiazepine withdrawal syndrome (BWS).
Dose
A milliliter of pure GBL metabolizes to the equivalent 1.65 g of NaGHB, the common form, so doses are measured in the single milliliter range, either taken all at once or sipped over the course of a night.
Legal status
Australia: GBL is not classified as a drug but as a health-endangering substance. Although legislation to enter into force on 1 April 2011 will make it possible to handle narcotics for industrial purposes will enable GBL and 1,4-Butanediol to be classified as controlled substances.[33]
Canada: GBL is a Controlled Substance under Schedule VI of the "Controlled Drugs and Substances Act" in Canada. Schedule VI of the "Controlled Drugs and Substances Act" requires vendors to collect information regarding purchases of GBL. The Act also prohibits the import and export of GBL into or out of Canada classifying it as either an indictable offense punishable with up to 10 years in prison or an offense punishable on summary conviction liable to imprisonment for up to eighteen months.[34] It is not illegal for an individual to possess GBL in Canada.
Germany: GBL is not listed in the narcotics law, but its distribution is controlled. Possession is not illegal, but may be punished according to the Medicines Act, when intended to be sold for human consumption or synthesis of GHB. In recent years, an increase of GBL consumption has been observed due to the prohibition of GHB.
Hong Kong SAR: GBL is a dangerous drug controlled under Schedule 1 of the Dangerous Drugs Ordinance, Cap.134 (with exemption clause at Paragraph 16D). Any person who is found to have in his possession of it not in accordance with this Ordinance can be liable, on conviction upon indictment, a fine of HK$1,000,000 and to imprisonment for 7 years.
Israel: GBL was classified as a proscribed substance from 2007.[35]
Netherlands: GBL is unlike GHB not listed in the narcotics law,[36] but its distribution is controlled. Possession is not illegal but may be punished according to the Medicines Act, when intended to be sold for human consumption or synthesis of GHB.[37]
Poland: GBL is classified as a drug. A license is mandatory for the manufacture, processing, reworking, importing, distribution of GBL.[38]
Russia: GBL has been classified as a psychotropic substance since 22 February 2012. Its trafficking is limited, and non-licensed selling, buying or any other use is punishable by imprisonment up to 20 years.
Sweden: GBL is not classified as a drug but as a health-endangering substance. Although recently passed legislation to enter into force on 1 April 2011 will make it possible to handle narcotics for industrial purposes will enable GBL and 1,4-Butanediol to be classified as controlled substances.[39]
United Kingdom: Because of their legitimate uses, regulation 4B of the 2001 regulations makes it lawful to import, export, produce, supply, offer to supply or possess GBL and 1,4-BD. Except where a person does so knowing or believing that they will be used for the purpose of human ingestion.[7][40]
United States: GBL is regulated as a List I controlled chemical. As a GHB analog, it is also treated as a controlled substance under Schedule I of the Controlled Substances Act if intended for human consumption.[41]
See also
References
- Merck Index, 12th Edition, 1632.
- Lide, David R., ed. (2009-06-03). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0. Retrieved 2011-07-18.
- "gamma-Butyrolactone_msds".
- Wolfgang Schwarz; Jürgen Schossig; Roland Rossbacher; Rolf Pinkos; Hartmut Höke (2019). "Butyrolactone". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_495.pub2.
- Vose, J.; Tighe, T.; Schwartz, M.; Buel, E. (2001). "Detection of gamma-butyrolactone (GBL) as a natural component in wine". Journal of Forensic Sciences. 46 (5): 1164–1167. doi:10.1520/JFS15116J. PMID 11569560.
- Elliott, S.; Burgess, V. (2005). "The presence of gamma-hydroxybutyric acid (GHB) and gamma-butyrolactone (GBL) in alcoholic and non-alcoholic beverages". Forensic Science International. 151 (2–3): 289–92. doi:10.1016/j.forsciint.2005.02.014. PMID 15939164.
- "A Change to the Misuse of Drugs Act 1971 : Control of GBL, 1,4-BD, BZP and related piperazine compounds, a further group of anabolic steroids and 2 non-steroidal agents, synthetic cannabinoid receptor agonists and oripavine" (PDF). Cite journal requires
|journal=(help) - Metsger, Leonid; Bittner, Shmuel (March 2000). "Autocatalytic Oxidation of Ethers with Sodium Bromate". Tetrahedron. 56 (13): 1905–1910. doi:10.1016/S0040-4020(00)00098-3.
- "Sandmeyer Reaction of GABA to GBL/GHB". Retrieved 2018-06-14.
- Micu, Alexandru (December 12, 2015). "New, fully recyclable and biodegradable plastic could change the world". ZME Science. ZME Science. Retrieved 2015-12-13.
- Hong, Miao; Chen, Eugene Y.-X. (2015). "Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone". Nature Chemistry. 8 (1): 42–49. doi:10.1038/nchem.2391. PMID 26673263.
- Hong, M.; Chen, E. Y.-X. (2016). "Towards Truly Sustainable Polymers: A Metal-Free Recyclable Polyester from Biorenewable Non-Strained γ-Butyrolactone". Angew. Chem. Int. Ed. 55 (13): 4188–93. doi:10.1002/anie.201601092. PMID 26934184.
- pg 149
- [Tianqi Niu, Jing Lu, Rahim Munir, Jianbo Li, Dounya Barrit, Xu Zhang, Hanlin Hu, Zhou Yang, Aram Amassian, Kui Zhao, and Shengzhong (Frank) Liu: "Stable High-Performance Perovskite Solar Cells via Grain Boundary Passivation", Adv. Mater. 2018, 1706576.]
- Kobilinsky, Lawrence (2011-11-29). Forensic Chemistry Handbook. p. 386. ISBN 978-0-471-73954-8.
- Teiber, J. F.; Draganov, D. I.; Du, B. N. L. (2003). "Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3". Biochemical Pharmacology. 66 (6): 887–96. doi:10.1016/S0006-2952(03)00401-5. PMID 12963475.
- "Gamma-butyrolactone (GBL) Pre-Review Report" (PDF). 4 Jun 2012.
- Van Cauter, E.; Plat, L.; Scharf, M. B.; Leproult, R.; Cespedes, S.; l'Hermite-Balériaux, M.; Copinschi, G. (1997). "Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men". Journal of Clinical Investigation. 100 (3): 745–753. doi:10.1172/JCI119587. PMC 508244. PMID 9239423.
- "Erowid GHB vault: FDA Warning about Gamma Butyrlactone". Erowid. 1998-11-21. Retrieved 2013-10-10.
- Meyer, Jerrold; Linda F. Quenzer (2005). Psychopharmacology: Drugs, the Brain and Behavior. Sinauer. p. 370. ISBN 978-0-87893-534-5.
- "USDOJ: U.S. Department of Justice Archive National Drug Intelligence Center" (PDF). Usdoj.gov. 2012-06-15. Retrieved 2014-01-22.
- Galloway, G. P.; Frederick-Osborne, S. L.; Seymour, R.; Contini, S. E.; Smith, D. E. (2000). "Abuse and therapeutic potential of gamma-hydroxybutyric acid". Alcohol. 20 (3): 263–269. doi:10.1016/S0741-8329(99)00090-7. PMID 10869868.
- "'There could be 100 comas in the year': Paris police chief reacts to rise of GBL, GHB overdoses in clubs". Resident Advisor. Retrieved 2018-04-19.
- "Drogue : " L'interdiction de vente au public du GBL n'a rien changé à la consommation "". Le Monde.fr (in French). Retrieved 2018-04-19.
- van Nieuwenhuijzen, PS; McGregor, IS (Aug 1, 2009). "Sedative and hypothermic effects of gamma-hydroxybutyrate (GHB) in rats alone and in combination with other drugs: assessment using biotelemetry". Drug and Alcohol Dependence. 103 (3): 137–47. doi:10.1016/j.drugalcdep.2009.03.004. PMID 19446408.
- Edwards, Richard (23 July 2009). "Coroner's 'Russian roulette' warning over GBL party drug". The Telegraph. The Telegraph. Retrieved May 1, 2012.
- "GBL/GHB". London Friend. Retrieved 18 August 2014.
- "GHB and GBL". GMFA. Retrieved 18 August 2014.
- Casciani, Dominic (23 December 2009). "GBL drug death identified by UK doctors". BBC News. Retrieved May 1, 2012.
- GHB addiction, GHB physical n psychological dependency levels Archived July 26, 2010, at the Wayback Machine
- Santos C, Olmedo RE (2017). "Sedative-Hypnotic Drug Withdrawal Syndrome: Recognition And Treatment". Emerg Med Pract. 19 (3): 1–20. PMID 28186869.
- Crew 2000 | GHB/ GBL Dependancy | | Drugs information, advice & support, Scotland, UK
- LAW AND JUSTICE LEGISLATION AMENDMENT (SERIOUS DRUG OFFENCES AND OTHER MEASURES) ACT 2005 NO. 129, 2005 - SCHEDULE 1
- Controlled Drugs and Substances Act (S.C. 1996, c. 19)
- section 7c of chapter B of part A of the 1st appendix of the Dangerous Drugs Act 1973
- "wetten.nl - Regeling - Opiumwet - BWBR0001941". Wetten.nl (in Dutch). 19 July 2019. Retrieved 19 July 2019.
- "Webwinkels gestopt met handel in GBL". Emerce (in Dutch). 9 December 2013. Retrieved 9 December 2013.
- https://www.gif.gov.pl/pl/decyzje-i-komunikaty/komunikaty/874,KOMUNIKAT-Nr-32016-GLOWNEGO-INSPEKTORA-FARMACEUTYCZNEGO.html
- Socialutskottets betänkande 2010/11:SoU5 - Riksdagen
- "UK Statutory Instrument 2011 No. 448". 2011-02-18.
- Information Bulletin: GHB Analogs; GBL, BD, GHV, and GVL
External links
- Erowid on GBL
- "The paint stripper drug that kills". BBC News. October 7, 2005.
- "All About GHB," a NIDA Neuroscience Consortium and OSPC "Cutting Edge" colloquium (27 June 2000 at the Doubletree hotel, Rockville, MD)