Inocoterone
Inocoterone (INN; developmental code name RU-29294) is a steroid-like nonsteroidal antiandrogen (NSAA) that was never marketed.[1][2] An acetate ester, inocoterone acetate, shows greater antiandrogen activity and was developed as a topical medication for the treatment of acne but showed only modest effectiveness in clinical trials and similarly was never marketed.[3]
| Antiandrogen | AR | PR | ER | GR | MR |
|---|---|---|---|---|---|
| Cyproterone acetate | 8–10 | 60 | <0.1 | 5 | 1 |
| Chlormadinone acetate | 5 | 175 | <0.1 | 38 | 1 |
| Megestrol acetate | 5 | 152 | <0.1 | 50 | 3 |
| Spironolactone | 7 | 0.4a | <0.1 | 2a | 182 |
| Trimethyltrienolone | 3.6 | <1 | <1 | <1 | <1 |
| Inocoterone | 0.8 | <0.1 | <0.1 | <0.1 | <0.1 |
| Inocoterone acetate | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Flutamide | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Hydroxyflutamide | 0.5–0.8 | <0.1 | <0.1 | <0.1 | <0.1 |
| Nilutamide | 0.5–0.8 | <0.1 | <0.1 | <0.1 | <0.1 |
| Bicalutamide | 1.8 | <0.1 | <0.1 | <0.1 | <0.1 |
| Notes: (1): Reference ligands (100%) were testosterone for the AR, progesterone for the PR, estradiol for the ER, dexamethasone for the GR, and aldosterone for the MR. (2): Tissues were rat prostate (AR), rabbit uterus (PR), mouse uterus (ER), rat thymus (GR), and rat kidney (MR). (3): Incubation times (0°C) were 24 hours (AR, a), 2 hours (PR, ER), 4 hours (GR), and 1 hour (MR). (4): Assay methods were different for bicalutamide for receptors besides the AR. Sources: See template. | |||||
 | |
| Clinical data | |
|---|---|
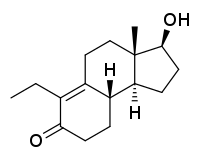
| Other names | RU-29294; 2,5-Seco-A-dinorestr-9-en-17β-ol-5-one |
| Routes of administration | Topical |
| Drug class | Nonsteroidal antiandrogen |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H24O2 |
| Molar mass | 248.366 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 686–. ISBN 978-1-4757-2085-3.
- Conn PM (23 July 2010). Techniques in Confocal Microscopy. Academic Press. pp. 215–. ISBN 978-0-12-384659-4.
- Annual Reports in Medicinal Chemistry. Academic Press. 2 September 1987. pp. 358–. ISBN 978-0-08-058366-2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.