Boldenone undecylenate
Boldenone undecylenate, or boldenone undecenoate, sold under the brand names Equipoise and Parenabol among others, is an androgen and anabolic steroid (AAS) medication which is used in veterinary medicine, mainly in horses.[2][3][4][5][6] It was formerly used in humans as well.[6] It is given by injection into muscle.[6]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Boldane, Equipoise, Parenabol, Vebonol, others |
| Other names | Boldenone undecenoate; Ba 29038; Boldenone 17β-undec-10-enoate; Δ1-Testosterone 17β-undec-10-enoate; 1-Dehydrotestosterone 17β-undec-10-enoate; Androsta-1,4-dien-17β-ol-3-one 17β-undec-10-enoate |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | Intramuscular: 14 days[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.032.734 |
| Chemical and physical data | |
| Formula | C30H44O3 |
| Molar mass | 452.679 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Side effects of boldenone undecylenate include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[6] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[6][7] It has strong anabolic effects and moderate androgenic effects, weak estrogenic effects, and no risk of liver damage.[6][7] Boldenone undecylenate is an androgen ester and a long-lasting prodrug of boldenone in the body.[6]
Boldenone undecylenate was introduced for medical use in the 1960s.[6] In addition to its medical use, boldenone undecylenate is used to improve physique and performance.[6] The drug is a controlled substance in the United States and its use is generally illicit.[6] It remains marketed for veterinary use in Australia and the United States.[6][5]
Uses
Veterinary
Boldenone undecylenate is used in veterinary medicine, mainly in horses.[6][5]
Clinical
Boldenone undecylenate was formerly used in clinical medicine in humans, but was discontinued.[6]
Side effects
Pharmacology
Pharmacodynamics
Boldenone undecylenate is a prodrug of boldenone, and hence is an agonist of the androgen receptor.[6] Boldenone has strong anabolic effects and moderate androgenic effects.[6] It also has low estrogenic activity and has little or no progestogenic activity.[6] In relation to the fact that it is not 17α-alkylated, boldenone and boldenone undecylenate have little or no risk of hepatotoxicity.[6]
Pharmacokinetics
Boldenone undecylenate is a prodrug of boldenone.[6] When administered via intramuscular injection, a depot is formed from which boldenone undecylenate is slowly released into the body and then transformed into boldenone.[6] Boldenone undecylenate is reported to possess a biological half-life of 14 days when administered by intramuscular injection.[1] Boldenone is a substrate for 5α-reductase and may be converted by this enzyme into 1-testosterone (δ1-dihydrotestosterone, δ1-DHT, dihydroboldenone) in tissues that express it such as the skin, hair follicles, and prostate gland.[6] However, its affinity for this enzyme is said to be extremely low.[6]
Chemistry
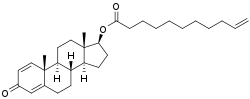
Boldenone undecylenate, or boldenone 17β-undec-10-enoate, is a synthetic androstane steroid and a derivative of testosterone.[2][3][6] It is the C17β undecylenate (undecenoate) ester of boldenone (δ1-testosterone, 1-dehydrotestosterone, or androsta-1,4-dien-17β-ol-3-one), which itself is the C1(2) dehydrogenated analogue of testosterone and a naturally occurring androgen found in the scent gland of Ilybius fenestratus (a species of aquatic beetle).[2][3][6] Boldenone is the non-17α-alkylated variant of metandienone (17α-methyl-δ1-testosterone).[2][3][6] An AAS related to boldenone undecylenate is quinbolone (δ1-testosterone 17β-cyclopentenyl enol ether).[2][3]
| Anabolic steroid | Structure | Ester | Relative mol. weight | Relative AAS contentb | Durationc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position | Moiety | Type | Lengtha | ||||||
| Boldenone undecylenate | C17β | Undecylenic acid | Straight-chain fatty acid | 11 | 1.58 | 0.63 | Long | ||
| Drostanolone propionate | C17β | Propanoic acid | Straight-chain fatty acid | 3 | 1.18 | 0.84 | Short | ||
| Metenolone acetate | C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.14 | 0.88 | Short | ||
| Metenolone enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.37 | 0.73 | Long | ||
| Nandrolone decanoate | C17β | Decanoic acid | Straight-chain fatty acid | 10 | 1.56 | 0.64 | Long | ||
| Nandrolone phenylpropionate | C17β | Phenylpropanoic acid | Aromatic fatty acid | – (~6–7) | 1.48 | 0.67 | Long | ||
| Trenbolone acetate | C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.16 | 0.87 | Short | ||
| Trenbolone enanthated | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | Long | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative androgen/anabolic steroid content by weight (i.e., relative androgenic/anabolic potency). c = Duration by intramuscular or subcutaneous injection in oil solution. d = Never marketed. Sources: See individual articles. | |||||||||
History
Boldenone was reportedly patented by Ciba in 1949, and esters of the compound were developed by the company in the 1950s and 1960s.[6] One such ester, boldenone undecylenate, was introduced for clinical use as an injectable AAS under the brand name Parenabol in the 1960s.[6] However, it was discontinued for use in humans in the late 1970s.[6] Squibb introduced boldenone undecylenate for veterinary use under the brand name Equipoise.[6] The medication has been used much more widely in veterinary medicine, in which it has been used mainly in horses, and remains in use today.[6][5]
Society and culture
Generic names
Boldenone undecylenate is the generic name of the drug and its USAN, while boldenone undecenoate is its BANM.[2][3][5]
Brand names
Boldenone undecylenate is or has been marketed under a number of brand names including Boldane, Equipoise, Parenabol, and Vebonol among others.[2][3][5][6]
Availability
Boldenone undecylenate remains marketed for veterinary use in Australia and the United States.[6][5]
Doping in sports
There are many known cases of doping in sports with boldenone undecylenate by professional athletes.
References
- Pedro Ruiz; Eric C. Strain (2011). Lowinson and Ruiz's Substance Abuse: A Comprehensive Textbook. Lippincott Williams & Wilkins. pp. 358–. ISBN 978-1-60547-277-5.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 640–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 131–. ISBN 978-3-88763-075-1.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 52–. ISBN 978-94-011-4439-1.
- "Boldenone - Drugs.com". Drugs.com. Retrieved 28 April 2017.
- William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 483–490. ISBN 978-0-9828280-1-4.
- Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.