Bisphenol F
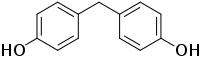
Bisphenol F (BPF; 4,4’-dihydroxydiphenylmethane) is a small aromatic organic compound with the chemical formula (HOC
6H
4)
2CH
2. It is related to bisphenol A through its basic structure, as both belong to the category of molecules known as bisphenols, which feature two phenol groups connected via a linking group. In BPF, the two aromatic rings are linked by a methylene connecting group.
 | |
| Names | |
|---|---|
| IUPAC name
4,4’-Methylenediphenol | |
| Other names
BPF; 4,4’-Dihydroxydiphenylmethane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.009.691 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H12O2 | |
| Molar mass | 200.237 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
BPF is used in the manufacturing of plastics and epoxy resins. It is used in the industry as a way of increasing the thickness and durability of materials.[1] Its use in this way is important in the production of tank and pipe linings, industrial floors, road and bridge deck toppings, structural adhesives, grouts, coatings and electrical varnishes.[2] BPF is also utilized in liners, lacquers, adhesives, plastics, and the coating of drinks and food cans.[1] Another use for BPF is in dental materials, where it can be found in restorative materials, liners, adhesives, oral prosthetic devices and tissue substitutes.[1]
Biological effects
Studies into the metabolism and excretion processes for the molecule have shown that the compound undergoes two primary phase II biotransformations to form the corresponding glucuronide and sulfate.[3][4][5] Different cell types have a bias towards which metabolite gets produced, with the human hepatoma cell line primarily metabolizing to the corresponding sulfate, and hepatocytes metabolizing to both the glucuronide and the sulfate compounds.[5] Additionally, phase I metabolism produces various hydroxylated metabolites of BPF, with the main metabolites being meta-hydroxylated BPF, ortho-hydroxylated BPF and dihydroxybenzophenone (DHB).[3][4] These metabolic pathways are P450 dependent.[4]
According to a study done on rats, the primary route of excretion of BPF and its metabolites is through the urine, with 43-54% of the dose being excreted in this manner.[2] In addition, 15-20% was excreted through the feces. The remainder of the administered dose was found throughout the rat, with it being found primarily in the digestive tract lumen and the liver. In pregnant rats, the BPF was also found in the uterus, placenta, amniotic fluid and fetuses, indicating that BPF is able to be absorbed into the reproductive tract and pass through the placental barrier in pregnant rats.[2]
Environmental contamination
BPF has been shown to be present in the environment and as a food contaminant.[3][6] But the main source for intake in the general population is probably mustard. It has been shown that BPF is built from natural ingredients during the manufacturing of mild mustard.[7] In this case BPF is a natural ingredient and not a contaminant from food contact material. This results in a low level, chronic exposure for humans. Due to this chronic exposure and the estrogenic effects that BPA has been shown to have, studies on BPF have occurred and are still occurring, to assess the effects that BPF has on living organisms.
The cytotoxicity and genotoxicity of BPF and some of its metabolites has been characterized, with BPF exhibiting an intermediate cytotoxicity.[3] BPF was not found to produce any genetic mutation when tested via an Ames test.[6] However, when human cell lines were tested and a Comet assay was conducted, BPF caused DNA fragmentation when introduced to the cells at non-cytotoxic concentrations.[6] In addition, another study found BPF to be genotoxic when introduced to Hep G2 cells.[3]
A literature review of in vivo studies of BPF found that four out of five studies yielded results that BPF is estrogenic, androgenic and thyroidogenic.[1] In addition, one study done in rats found the greatest effect of BPF to be liver toxicity.[8] In vitro studies of BPF showed effects of cytotoxicity, cellular dysfunction, DNA damage and chromosomal aberrations.[1]
BPF prenatal exposure is also associated with impaired cognitive functions in studies on mother-child pairs from the Swedish Environmental Longitudinal (SELMA Studies).[9]
References
- Rochester, Johanna Ruth; Bolden, Ashley Louise (2015). "Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes". Environmental Health Perspectives. 123 (7): 643–50. doi:10.1289/ehp.1408989. PMC 4492270. PMID 25775505.
- Cabaton, Nicolas; Chagnon, Marie-Christine; Lhuguenot, Jean-Claude; Cravedi, Jean-Pierre; Zalko, Daniel (2006-12-27). "Disposition and metabolic profiling of bisphenol F in pregnant and nonpregnant rats". Journal of Agricultural and Food Chemistry. 54 (26): 10307–10314. doi:10.1021/jf062250q. ISSN 0021-8561. PMID 17177575.
- Audebert, Marc; Dolo, L.; Perdu, E.; Cravedi, J.-P.; Zalko, D. (2011-06-09). "Use of the γH2AX assay for assessing the genotoxicity of bisphenol A and bisphenol F in human cell lines". Archives of Toxicology. 85 (11): 1463–1473. doi:10.1007/s00204-011-0721-2. ISSN 0340-5761. PMID 21656223.
- Cabaton, Nicolas; Zalko, Daniel; Rathahao, Estelle; Canlet, Cécile; Delous, Georges; Chagnon, Marie-Christine; Cravedi, Jean-Pierre; Perdu, Elisabeth (2008-10-01). "Biotransformation of bisphenol F by human and rat liver subcellular fractions". Toxicology in Vitro. 22 (7): 1697–1704. doi:10.1016/j.tiv.2008.07.004. ISSN 0887-2333. PMID 18672047.
- Dumont, Coralie; Perdu, Elisabeth; Sousa, Georges de; Debrauwer, Laurent; Rahmani, Roger; Cravedi, Jean-Pierre; Chagnon, Marie-Christine (2011-10-01). "Bis(hydroxyphenyl)methane—bisphenol F—metabolism by the HepG2 human hepatoma cell line and cryopreserved human hepatocytes". Drug and Chemical Toxicology. 34 (4): 445–453. doi:10.3109/01480545.2011.585651. ISSN 0148-0545. PMID 21770713.
- Cabaton, Nicolas; Dumont, Coralie; Severin, Isabelle; Perdu, Elisabeth; Zalko, Daniel; Cherkaoui-Malki, Mustapha; Chagnon, Marie-Christine (2009-01-08). "Genotoxic and endocrine activities of bis(hydroxyphenyl)methane (bisphenol F) and its derivatives in the HepG2 cell line". Toxicology. 255 (1–2): 15–24. doi:10.1016/j.tox.2008.09.024. ISSN 0300-483X. PMID 18973785.
- Zoller, O.; Brüschweiler, B. J.; Magnin, R.; Reinhard, H.; Rhyn, P.; Rupp, H.; Zeltner, S.; Felleisen, R. (2015-11-23). "Natural occurrence of bisphenol F in mustard". Food Additives & Contaminants: Part A. 33 (1): 137–146. doi:10.1080/19440049.2015.1110623. ISSN 1944-0049. PMID 26555822.
- Higashihara, Nobuhiko; Shiraishi, Keiji; Miyata, Katusi; Oshima, Yutaka; Minobe, Yasushi; Yamasaki, Kanji (2007-12-01). "Subacute oral toxicity study of bisphenol F based on the draft protocol for the "Enhanced OECD Test Guideline no. 407"". Archives of Toxicology. 81 (12): 825–832. doi:10.1007/s00204-007-0223-4. ISSN 0340-5761. PMID 17628788.
- Tanner, Eva M.; Hallerbäck, Maria Unenge; Wikström, Sverre; Lindh, Christian; Kiviranta, Hannu; Gennings, Chris; Bornehag, Carl-Gustaf (2020-01-01). "Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven". Environment International. 134: 105185. doi:10.1016/j.envint.2019.105185. ISSN 0160-4120.