List of fentanyl analogues
This is a list of fentanyl analogues, including both compounds developed by pharmaceutical companies for legitimate medical use, and those which have been sold as designer drugs and reported to national drug control agencies such as the DEA, or transnational agencies such as the EMCDDA and UNODC.[1][2][3][4][5][6] This is not a comprehensive listing of fentanyl analogues, as more than 1400 compounds from this family have been described in the scientific and patent literature,[7][8][9][10][11] but it includes all notable compounds that have reached late-stage human clinical trials, or which have been identified as having been sold as designer drugs.
In the United States, the Drug Enforcement Administration placed the broadly defined class of "Fentanyl-Related Substances" on the list of Schedule I drugs in 2018, making it illegal to manufacture, distribute, or possess fentanyl analogs.[12]
Chemical structures of various fentanyl analogues
| Chemical structure | Common name | Chemical name | CAS number |
|---|---|---|---|
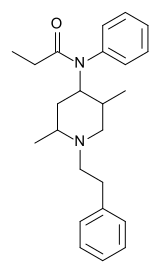 |
2,5-Dimethylfentanyl | N-[2,5-Dimethyl-1-(2-phenylethyl)piperidin-4-yl]-N-phenylpropanamide | 42045-97-6 |
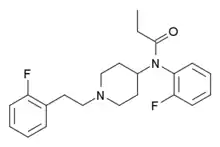 |
2,2'-Difluorofentanyl | N-(2-Fluorophenyl)-N-[1-(2-[2-fluorophenyl]ethyl)-4-piperidinyl]-propanamide | |
 |
3-Allylfentanyl | N-[(3S,4R)-1-Phenethyl-3-prop-2-enylpiperidin-4-yl]-N-phenylpropanamide | 82208-84-2 |
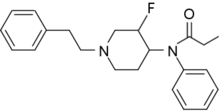 |
3-Fluorofentanyl (NFEPP) | N-(3-Fluoro-1-phenethylpiperidin-4-yl)-N-phenylpropionamide | 1422952-84-8 |
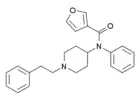 |
3-Furanylfentanyl (3FUF) | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]furan-3-carboxamide | 101343-82-2 |
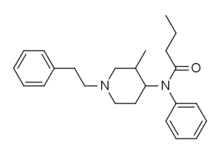 |
3-Methylbutyrfentanyl | N-[3-Methyl-1-(2-phenylethyl)piperidin-4-yl]-N-phenylbutanamide | 97605-09-9 |
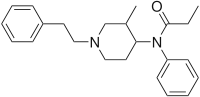 |
3-Methylfentanyl (3-MF) | N-(3-methyl-1-phenethyl-4-piperidyl)-N-phenyl-propanamide | 42045-86-3 |
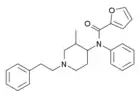 |
3-Methylfuranylfentanyl[13] (3MFUF, TMFUF) | N-Phenyl-N-[3-methyl-1-(2-phenylethyl)piperidin-4-yl]furan-2-carboxamide | |
 |
3-Methylthiofentanyl | N-{3-Methyl-1-[2-(2-thienyl)ethyl]piperidin-4-yl}-N-phenylpropanamide | 86052-04-2 |
 |
3-Phenylpropanoylfentanyl | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]-3-phenylpropanamide | 79279-02-0 |
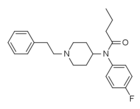 |
4-Fluorobutyrfentanyl (4-FBF) | N-(4-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-butanamide | 244195-31-1 |
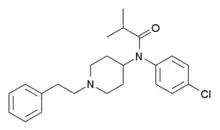 |
4-Chloroisobutyrylfentanyl (4-CIBF) [14] | 2-Methyl-N-(4-chlorophenyl)-N-[1-(1-phenylpropan-2-yl)piperidin-4-yl]propanamide | 244195-34-4 |
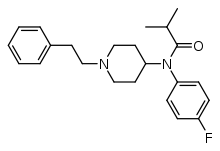 |
4-Fluoroisobutyrfentanyl (4-FIBF) | N-(4-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-isobutanamide | 244195-32-2 |
 |
4-Fluorofentanyl | N-(4-fluorophenyl)-N-[1-(2-phenylethyl)piperidin-4-yl]propanamide | 90736-23-5 |
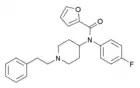 |
para-fluorofuranylfentanyl[15] (p-F-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(4-fluorophenyl)furan-2-carboxamide | 1802489-71-9 |
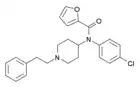 |
para-chlorofuranylfentanyl (p-Cl-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(4-chlorophenyl)furan-2-carboxamide | |
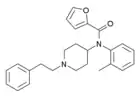 |
ortho-methylfuranylfentanyl (o-Me-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(o-tolyl)furan-2-carboxamide | |
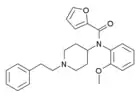 |
ortho-methoxyfuranylfentanyl (o-MeO-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(2-methoxyphenyl)furan-2-carboxamide | |
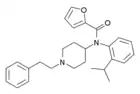 |
ortho-isopropylfuranylfentanyl (o-iPr-Fu-F) | N-(1-phenethylpiperidin-4-yl)-N-(2-isopropylphenyl)furan-2-carboxamide | |
 |
4-Phenylfentanyl | N-Phenyl-N-[4-phenyl-1-(2-phenylethyl)piperidin-4-yl]propanamide | 120448-97-7 |
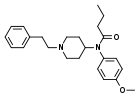 |
4-Methoxybutyrfentanyl | N-(4-Methoxyphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-butanamide | 2088842-68-4 |
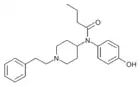 |
para-Hydroxy‐butyrylfentanyl [16] | N-(4-hydroxyphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-butanamide | |
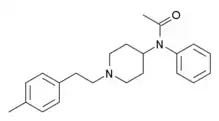 |
4-Methylphenethylacetylfentanyl [17] | N-[1-[2-(4-Methylphenyl)ethyl]-4-piperidinyl]-N-phenylacetamide | 1071703-95-1 |
 |
Acrylfentanyl | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]prop-2-enamide | 82003-75-6 |
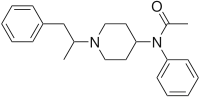 |
α-Methylacetylfentanyl | N-Phenyl-N-[1-(1-phenylpropan-2-yl)-4-piperidyl]acetamide | 101860-00-8 |
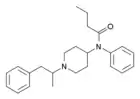 |
α-Methylbutyrfentanyl | N-phenyl-N-[1-(1-phenylpropan-2-yl)-4-piperidyl]butanamide | 244195-36-6 |
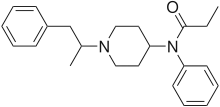 |
α-Methylfentanyl (AMF) | N-phenyl-N-[1-(1-phenylpropan-2-yl)-4-piperidyl]propanamide | 79704-88-4 |
 |
α-Methylthiofentanyl | N-Phenyl-N-[1-(1-thiophen-2-ylpropan-2-yl)-4-piperidyl]propanamide | 103963-66-2 |
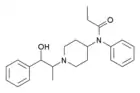 |
α-Methyl-β-hydroxyfentanyl[18] | N-[1-(1-hydroxy-1-phenylpropan-2-yl)piperidin-4-yl]-N-phenylpropanamide | |
 |
Acetylfentanyl | N-(1-Phenethylpiperidin-4-yl)-N-phenylacetamide | 3258-84-2 |
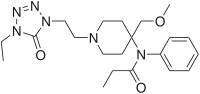 |
Alfentanyl | N-{1-[2-(4-ethyl-5-oxo-4,5-dihydro-1H-1,2,3,4-tetrazol-1-yl)ethyl]-4-(methoxymethyl)piperidin-4-yl}-N-phenylpropanamide | 71195-58-9 |
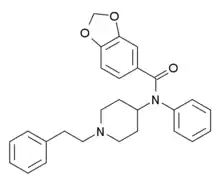 |
Benzodioxolefentanyl [19] | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]-2H-1,3-benzodioxole-5-carboxamide | 2306823-01-6 |
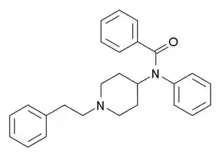 |
Benzoylfentanyl | N-(1-Phenethylpiperidin-4-yl)-N-phenylbenzamide | 2309383-15-9 |
 |
Benzylfentanyl | N-(1-Benzylpiperidin-4-yl)-N-phenylpropanamide | 1474-02-8 |
 |
β-Hydroxyfentanyl | N-[1-(2-Hydroxy-2-phenylethyl)piperidin-4-yl]-N-phenylpropanamide | 78995-10-5 |
 |
β-Hydroxythiofentanyl | N-{1-[2-Hydroxy-2-(thiophen-2-yl)ethyl]piperidin-4-yl}-N-phenylpropanamide | 1474-34-6 |
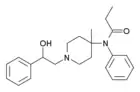 |
β-Hydroxy-4-methylfentanyl | N-[1-(2-hydroxy-2-phenylethyl)-4-methylpiperidin-4-yl]-N-phenylpropanamide | |
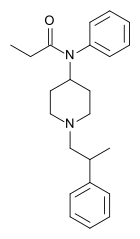 |
β-Methylfentanyl | N-Phenyl-N-[1-(2-phenylpropyl)piperidin-4-yl]propanamide | 79146-56-8 |
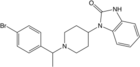 |
Brorphine [20] | 1-{1-[1-(4-bromophenyl)ethyl]piperidin-4-yl}-1,3-dihydro-2H-benzimidazol-2-one | 2244737-98-0 |
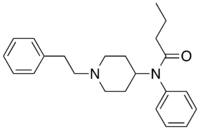 |
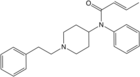
Butyrfentanyl (Bu-F, BUF) | N-(1-(2-Phenylethyl)-4-piperidinyl)-N-phenylbutyramide | 1169-70-6 |
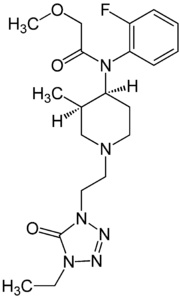 |
Brifentanyl | N-[(3R,4S)-1-[2-(4-Ethyl-5-oxotetrazol-1-yl)ethyl] -3-methylpiperidin-4-yl]-N-(2-fluorophenyl)-2-methoxyacetamide | 101345-71-5 |
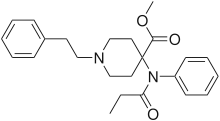 |
Carfentanyl | Methyl 1-(2-phenylethyl)-4-[phenyl(propanoyl)amino]piperidine-4-carboxylate | 59708-52-0 |
 |
Crotonylfentanyl | N-Phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]-2-butenamide | 760930-59-4 |
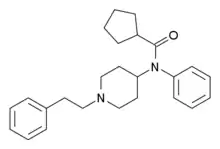 |
Cyclopentylfentanyl | N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]cyclopentanecarboxamide | 2088918-01-6 |
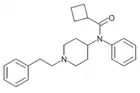 |
Cyclobutylfentanyl [21] | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-phenylcyclobutanecarboxamide | |
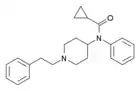 |
Cyclopropylfentanyl | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-phenylcyclopropanecarboxamide | 1169-68-2 |
.png.webp) |
EAZ-91-05 | (quinuclidin-3-yl) 4-[phenyl(propanoyl)amino]-1-[2-(indol-3-yl)ethyl]piperidine-4-carboxylate | |
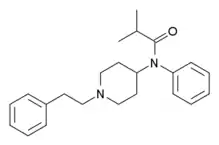 |
Isobutyrylfentanyl | 2-methyl-N-phenyl-N-[1-(1-phenylpropan-2-yl)piperidin-4-yl]propanamide | 119618-70-1 |
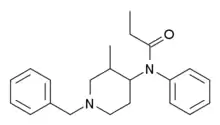 |
Isofentanyl | N-(1-Benzyl-3-methylpiperidin-4-yl)-N-phenylpropanamide | 79278-40-3 |
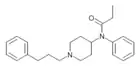 |
Homofentanyl (N-phenylpropylnorfentanyl) [22] | N-phenyl-N-[1-(3-phenylpropyl)piperidin-4-yl]propanamide | 59708-54-2 |
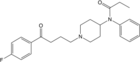 |
Hybrid neuroleptanalgesic containing features of both fentanyl & haloperidol[23] | N-phenyl-N-{1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl}propanamide | |
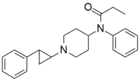 |
trans-phenylcyclopropyl-norfentanyl | 1-(trans-2-Phenylcyclopropyl)-4-(N-propionylanilino)piperidine. | 102504-49-4 |
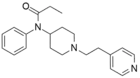 |
Pyridylfentany[24] | N-phenyl-N-[1-(2-pyridin-4-ylethyl)piperidin-4-yl]propanamide | 1443-41-0 |
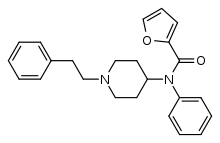 |
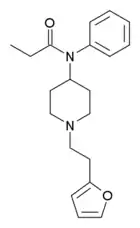
Furanylfentanyl (Fu-F, FUF) | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]furan-2-carboxamide | 101345-66-8 |
 |
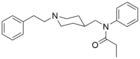
Furanylethylfentanyl (FUEF) | N-[1-[2-(2-furanyl)ethyl]-4-piperidinyl]-N-phenyl-propanamide | 802544-02-1 |
 |
Fentanyl 4-methylene analogue (WO 2007/093603) | N-phenyl-N-{[1-(2-phenylethyl)piperidin-4-yl]methyl}propanamide | |
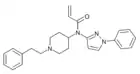 |
IQMF-4[25] | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-(1-phenylpyrazol-3-yl)prop-2-enamide | |
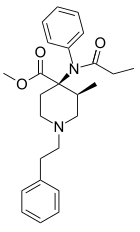 |
Lofentanyl | methyl (3S,4R)-3-methyl-1-(2-phenylethyl)-4-[phenyl(propionyl)amino]piperidine-4-carboxylate | 61380-40-3 |
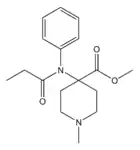 |
N-Methylnorcarfentanyl | methyl 1-methyl-4-(N-phenylpropanamido)piperidine-4-carboxylate | 59708-50-8 |
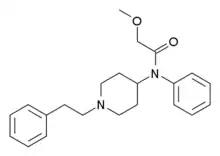 |
Methoxyacetylfentanyl (MAF) | 2-Methoxy-N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide | 101345-67-9 |
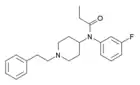 |
meta-fluorofentanyl | N-(3-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-propanamide | 90736-22-4 |
 |
Mirfentanyl | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-pyrazin-2-yl-2-furamide | 117523-47-4 |
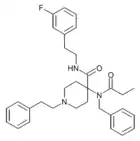 |
MP135[26] | N-[4-{[2-(3-fluorophenyl)ethyl]carboxamido}-1-(2-phenylethyl)piperidin-4-yl]-N-benzylpropanamide | |
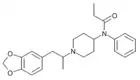 |
N-(MDA)-fentanyl[27] | N-(1-(1-(benzo[d][1,3]dioxol-5-yl)propan-2-yl)piperidin-4-yl)-N-phenylpropionamide | |
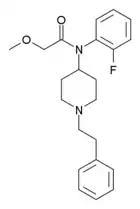 |
Ocfentanyl | N-(2-Fluorophenyl)-2-methoxy-N-[1-(2-phenylethyl)piperidin-4-yl]acetamide | 101343-69-5 |
 |
Ohmefentanyl | N-[1-(2-hydroxy-2-phenylethyl)-3-methylpiperidin-4-yl]-N-phenylpropanamide | 78995-14-9 |
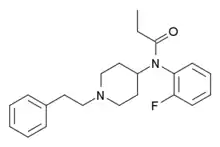 |
Orthofluorofentanyl | N-(2-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-propanamide | 910616-29-4 |
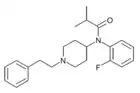 |
ortho-Fluoroisobutyrylfentanyl | N-(2-Fluorophenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-2-methylpropanamide | 2351142-33-9 |
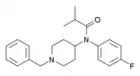 |
Parafluoroisobutyrylbenzylfentanyl | N-[(1-benzylpiperidin-4-yl)methyl]-N-(4-fluorophenyl)-2-methylpropanamide | |
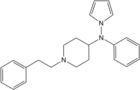 |
Pyrrole-fentanyl [28] | N-[1-(2-Phenylethyl)piperidin-4-yl]-N-pyrrol-1-ylpropanamide | |
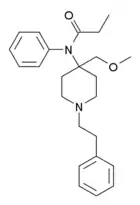 |
R-30490 | N-[4-(Methoxymethyl)-1-(2-phenylethyl)piperidin-4-yl]-N-phenylpropanamide | 60618-49-7 |
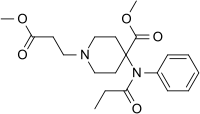 |
Remifentanyl | methyl 1-(3-methoxy-3-oxopropyl)-4-(N-phenylpropanamido)piperidine-4-carboxylate | 132875-61-7 |
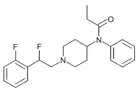 |
RR49 [29] | N-{1-[2-Fluoro-2-(2-fluorophenyl)ethyl]piperidin-4-yl}-N-phenylpropanamide | |
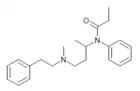 |
Secofentanyl [30] | N-phenyl-N-{4-[methyl(2-phenylethyl)amino]butan-2-yl}propanamide | |
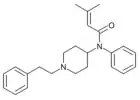 |
Senecioylfentanyl [31] | N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]-3-methylbut-2-enamide | |
 |
Sufentanil | N-[4-(Methoxymethyl)-1-(2-thiofuran-2-ylethyl)-4-piperidyl]-N-phenylpropanamide | 56030-54-7 |
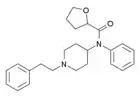 |
Tetrahydrofuranylfentanyl | N-Phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]oxolane-2-carboxamide | 2142571-01-3 |
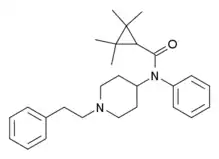 |
Tetramethylcyclopropylfentanyl[32] | 2,2,3,3-Tetramethyl-N-(1-phenethylpiperidin-4-yl)-N-phenylcyclopropane-1-carboxamide | 2309383-11-5 |
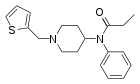 |
Thenylfentanyl | N-phenyl-N-{[1-(thiophen-2-ylmethyl)piperidin-4-yl]methyl}propanamide | 117332-93-1 |
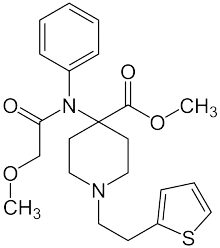 |
Thiafentanil | methyl 4-(N-(2-methoxyacetyl)anilino)-1-(2-thiophen-2-ylethyl)piperidine-4-carboxylate | 101345-60-2 |
 |
Thiofentanyl | N-phenyl-N-{1-[2-(2-thienyl)ethyl]piperidin-4-yl}propanamide | 1165-22-6 |
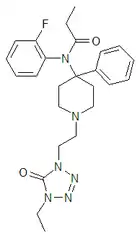 |
Trefentanyl | N-{1-[2-(4-ethyl-5-oxo-4,5-dihydro-1H-tetrazol-1-yl)ethyl]-4-phenylpiperidin-4-yl}-N-(2-fluorophenyl)propanamide | 120656-93-1 |
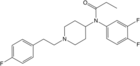 |
Trifluorofentanyl [33] | N-(3,4-difluorophenyl)-N-[1-(2-[4-fluorophenyl]ethyl)-4-piperidinyl]-propanamide | |
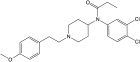 |
3,4-dichloro-4''-methoxyfentanyl | N-(3,4-dichlorophenyl)-N-[1-(2-[4-methoxyphenyl]ethyl)-4-piperidinyl]-propanamide | |
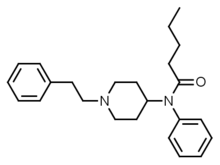 |
Valerylfentanyl (VF) | N-(1-(2-Phenylethyl)-4-piperidinyl)-N-phenylpentylamide | 122882-90-0 |
Analogue controls
Several jurisdictions have implemented analogue law controls of fentanyl analogues in an attempt to pre-emptively ban novel derivatives before they appear on the market. One representative example is the New Zealand provisions enacted in 1988 in response to the first wave of fentanyl derivatives. This bans a set of structures as follows;
"Fentanyl analogues, in which the N-[1-(2-phenethyl)-4-piperidyl]aniline nucleus has additional radicals, either alone or in combination, attached as follows:
(a) an acetyl, propionyl, butenoyl or butanoyl radical, attached to the aniline nitrogen atom:
(b) 1 or more alkyl radicals, with up to 10 carbon atoms in total, attached to the ethyl moiety:
(c) any combination of up to 5 alkyl radicals and/or alkoxy radicals (each with up to 6 carbon atoms, including cyclic radicals) and/or halogen radicals, attached to each of the benzene rings."[34]
A more recent and somewhat broader example was introduced into US Federal legislation in 2018, covering the following structures;
"...fentanyl-related substances includes any substance not otherwise controlled in any schedule...that is structurally related to fentanyl by one or more of the following modifications:
- Replacement of the phenyl portion of the phenethyl group by any monocycle, whether or not further substituted in or on the monocycle;
- substitution in or on the phenethyl group with alkyl, alkenyl, alkoxyl, hydroxyl, halo, haloalkyl, amino or nitro groups;
- substitution in or on the piperidine ring with alkyl, alkenyl, alkoxyl, ester, ether, hydroxyl, halo, haloalkyl, amino or nitro groups;
- replacement of the aniline ring with any aromatic monocycle whether or not further substituted in or on the aromatic monocycle; and/or
- replacement of the N-propionyl group by another acyl group."[35]
See also
References
- Mounteney J, Giraudon I, Denissov G, Griffiths P (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". The International Journal on Drug Policy. 26 (7): 626–31. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
- Zawilska JB (2017). "An Expanding World of Novel Psychoactive Substances: Opioids". Frontiers in Psychiatry. 8: 110. doi:10.3389/fpsyt.2017.00110. PMC 5492455. PMID 28713291.
- "Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens" (PDF). United Nations Office on Drugs and Crime. November 2017.
- Vardanyan RS, Hruby VJ (March 2014). "Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications". Future Medicinal Chemistry. 6 (4): 385–412. doi:10.4155/fmc.13.215. PMC 4137794. PMID 24635521.
- Armenian P, Vo KT, Barr-Walker J, Lynch KL (May 2018). "Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review". Neuropharmacology. 134 (Pt A): 121–132. doi:10.1016/j.neuropharm.2017.10.016. PMID 29042317. S2CID 21404877.
- Fentanyl-related substances with no known legitimate uses. International Narcotics Control Board, 24 June 2018
- Bagley JR, Kudzma LV, Lalinde NL, Colapret JA, Huang BS, Lin BS, et al. (July 1991). "Evolution of the 4-anilidopiperidine class of opioid analgesics". Medicinal Research Reviews. 11 (4): 403–36. doi:10.1002/med.2610110404. PMID 1875771. S2CID 33000913.
- Skulska A, Kała M, Parczewski A (2004). "Fentanyl and its analogs in the forensic laboratory. Medical and analytical problems" (PDF). Probl Forensic Sci. 59: 127–42.
- Misailidi N, Papoutsis I, Nikolaou P, Dona A, Spiliopoulou C, Athanaselis S (January 2018). "Fentanyls continue to replace heroin in the drug arena: the cases of ocfentanil and carfentanil". Forensic Toxicology. 36 (1): 12–32. doi:10.1007/s11419-017-0379-4. PMC 5754389. PMID 29367860.
- Wilde M, Pichini S, Pacifici R, Tagliabracci A, Busardò FP, Auwärter V, Solimini R (2019). "Metabolic Pathways and Potencies of New Fentanyl Analogs". Frontiers in Pharmacology. 10: 238. doi:10.3389/fphar.2019.00238. PMC 6461066. PMID 31024296.
- Sofalvi S, Lavins ES, Brooker IT, Kaspar CK, Kucmanic J, Mazzola CD, et al. (October 2019). "Unique Structural/Stereo-Isomer and Isobar Analysis of Novel Fentanyl Analogues in Postmortem and DUID Whole Blood by UHPLC-MS-MS". Journal of Analytical Toxicology. 43 (9): 673–687. doi:10.1093/jat/bkz056. PMID 31504606.
- Drug Enforcement Administration, Department of Justice (February 2018). "Schedules of Controlled Substances: Temporary Placement of Fentanyl-Related Substances in Schedule I. Temporary amendment; temporary scheduling order". Federal Register. 83 (25): 5188–92. PMID 29932611.
- Lalinde N, Moliterni J, Wright D, Spencer HK, Ossipov MH, Spaulding TC, Rudo FG (October 1990). "Synthesis and pharmacological evaluation of a series of new 3-methyl-1,4-disubstituted-piperidine analgesics". Journal of Medicinal Chemistry. 33 (10): 2876–82. doi:10.1021/jm00172a032. PMID 2170652.
- Le AD, Alzghari SK (October 2019). "Systematic review of the clinical consequences of butyrfentanyl and corresponding analogues". Interdisciplinary Toxicology. 12 (2): 83–88. doi:10.2478/intox-2019-0009. PMC 7071838. PMID 32206028.
- Vincenti F, Montesano C, Di Ottavio F, Gregori A, Compagnone D, Sergi M, Dorrestein P (2020). "Molecular Networking: A Useful Tool for the Identification of New Psychoactive Substances in Seizures by LC-HRMS". Frontiers in Chemistry. 8: 572952. doi:10.3389/fchem.2020.572952. PMC 7723841. PMID 33324608.
- Oldenhof S, Ten Pierick A, Bruinsma J, Eustace S, Hulshof J, van den Berg J, Hoitink M (January 2020). "Identification of a novel fentanyl analog: p-Hydroxy-butyrylfentanyl". Drug Testing and Analysis. 12 (1): 152–155. doi:10.1002/dta.2695. PMID 31518047.
- Moore JM, Allen AC, Cooper DA, Carr SM (July 1986). "Determination of fentanyl and related compounds by capillary gas chromatography with electron capture detection". Analytical Chemistry. 58 (8): 1656–60. doi:10.1021/ac00121a013. PMID 3752503.
- Barceloux DG (2012). Medical Toxicology of Drug Abuse: Synthesized Chemicals and Psychoactive Plants. Wiley. ISBN 978-0471727606.
- Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM (June 2019). "Opioid-like antinociceptive and locomotor effects of emerging fentanyl-related substances". Neuropharmacology. 151: 171–179. doi:10.1016/j.neuropharm.2019.03.023. PMID 30904478. S2CID 84182661.
- Kennedy NM, Schmid CL, Ross NC, Lovell KM, Yue Z, Chen YT, et al. (October 2018). "Optimization of a Series of Mu Opioid Receptor (MOR) Agonists with High G Protein Signaling Bias". Journal of Medicinal Chemistry. 61 (19): 8895–8907. doi:10.1021/acs.jmedchem.8b01136. PMC 6386185. PMID 30199635.
- Elbardisy HM, Foster CW, Cumba L, Antonides LH, Gilbert N, Schofield CJ, et al. (2019). "Analytical determination of heroin, fentanyl and fentalogues using high-performance liquid chromatography with diode array and amperometric detection". Analytical Methods. 11 (8): 1053–63. doi:10.1039/C9AY00009G.
- Cooper D, Jacob M, Allen A (April 1986). "Identification of fentanyl derivatives". Journal of Forensic Sciences. 31 (2): 511–28. doi:10.1520/JFS12283J. PMID 3711827.
- US granted 3161637, Janssen PA, "1-(gamma-aroyl-propyl)-4-(nu-arylcarbonyl amino) piperidines and related compounds", issued 15 December 1964, assigned to Janssen Resarch Labs
- Robertson TB, Antonides LH, Gilbert N, Benjamin SL, Langley SK, Munro LJ, Sutcliffe OB, Mewis RE (December 2019). "Hyperpolarization of Pyridyl Fentalogues by Signal Amplification By Reversible Exchange (SABRE)". ChemistryOpen. 8 (12): 1375–1382. doi:10.1002/open.201900273. PMC 6892445. PMID 31844604.
- Goicoechea C, Sánchez E, Cano C, Jagerovic N, Martín MI (October 2008). "Analgesic activity and pharmacological characterization of N-[1-phenylpyrazol-3-yl]-N-[1-(2-phenethyl)-4-piperidyl] propenamide, a new opioid agonist acting peripherally". European Journal of Pharmacology. 595 (1–3): 22–9. doi:10.1016/j.ejphar.2008.07.052. PMID 18706410.
- Faouzi A, Uprety R, Gomes I, Massaly N, Keresztes AI, Le Rouzic V, Gupta A, Zhang T, Yoon HJ, Ansonoff M, Allaoa A, Pan YX, Pintar J, Morón JA, Streicher JM, Devi LA, Majumdar S (November 2020). "Synthesis and Pharmacology of a Novel μ-δ Opioid Receptor Heteromer-Selective Agonist Based on the Carfentanyl Template". Journal of Medicinal Chemistry. 63 (22): 13618–13637. doi:10.1021/acs.jmedchem.0c00901. PMID 33170687.
- W De Silva I, Couch AN, Verbeck GF (December 2020). "Paper Spray Mass Spectrometry Utilized with a Synthetic Microporous Polyolefin Silica Matrix Substrate in the Rapid Detection and Identification of More than 190 Synthetic Fentanyl Analogs". Journal of the American Society for Mass Spectrometry. doi:10.1021/jasms.0c00250. PMID 33296202.
- US 4452803, Effland RC, Klein JT, "Pyrrolylaminopiperidines.", issued 19 September 1983
- Rosas R, Huang XP, Roth BL, Dockendorff C (September 2019). "β-Fluorofentanyls Are pH-Sensitive Mu Opioid Receptor Agonists". ACS Medicinal Chemistry Letters. 10 (9): 1353–1356. doi:10.1021/acsmedchemlett.9b00335. PMC 6746189. PMID 31531209.
- Ivanović MD, Mićović I, Vučković S, Prostran M, Todorović Z, Ivanović E, Kiricojević V, Đorđević JB, Došen-Mićović L (2004). "The synthesis and pharmacological evaluation of (+/-)-2, 3-seco-fentanyl analogues". Journal of the Serbian Chemical Society. 69 (11): 955–68.
- Bergh MS, Bogen IL, Nerem E, Wohlfarth A, Wilson SR, Øiestad ÅM (2021). "Discovering the major metabolites of the three novel fentanyl analogues 3-methylcrotonylfentanyl, furanylbenzylfentanyl, and 4-fluorocyclopropylbenzylfentanyl for forensic case work". Forensic Toxicology. 39: 167–178. doi:10.1007/s11419-020-00560-9.
- Åstrand A, Vikingsson S, Jakobsen I, Björn N, Kronstrand R, Gréen H (August 2020). "Activation of the μ-opioid receptor by alicyclic fentanyls: Changes from high potency full agonists to low potency partial agonists with increasing alicyclic substructure". Drug Testing and Analysis. doi:10.1002/dta.2906. PMID 32749741.
- US abandoned 2011046180, Peters D, Eriksen BL, Munro G, Nielsen EØ, "N-aryl-n-piperidin-4-yl-propionamide derivatives and their use as opioid receptor ligands", assigned to Neurosearch AS
- "Schedule 3 Class C Controlled Drugs; Part 7: Fentanyl analogues". New Zealand Misuse of Drugs Act 1975.
- "Schedules of Controlled Substances: Temporary Placement of Fentanyl-Related Substances in Schedule I". United States Federal Register. 6 February 2018.
