Pain in amphibians
Pain is an aversive sensation and feeling associated with actual, or potential, tissue damage.[1] It is widely accepted by a broad spectrum of scientists and philosophers that non-human animals can perceive pain, including pain in amphibians.

Pain is a complex mental state, with a distinct perceptual quality but also associated with suffering, which is an emotional state. Because of this complexity, the presence of pain in non-human animals cannot be determined unambiguously using observational methods, but the conclusion that animals experience pain is often inferred on the basis of likely presence of phenomenal consciousness which is deduced from comparative brain physiology as well as physical and behavioural reactions.[2][3]
Amphibians, particularly anurans, fulfill several physiological and behavioural criteria proposed as indicating that non-human animals may experience pain. These fulfilled criteria include a suitable nervous system and sensory receptors, opioid receptors and reduced responses to noxious stimuli when given analgesics and local anaesthetics, physiological changes to noxious stimuli, displaying protective motor reactions, exhibiting avoidance learning and making trade-offs between noxious stimulus avoidance and other motivational requirements.
Pain in amphibians has societal implications including their exposure to pollutants, (preparation for) cuisine (e.g. Frogs legs) and amphibians used in scientific research.
Several scientists and scientific groups have expressed the belief that amphibians can feel pain, however, this remains somewhat controversial due to differences in brain structure and the nervous system compared with other vertebrates.

Background
The possibility that amphibians and other non-human animals may experience pain has a long history. Initially, pain in non-human animals was based around theoretical and philosophical argument, but more recently has turned to scientific investigation.
Philosophy

The idea that non-human animals might not feel pain goes back to the 17th-century French philosopher, René Descartes, who argued that animals do not experience pain and suffering because they lack consciousness.[4][5][6] In 1789, the British philosopher and social reformist, Jeremy Bentham, addressed in his book An Introduction to the Principles of Morals and Legislation the issue of our treatment of animals with the following often quoted words: "The question is not, Can they reason? nor, can they talk? but, Can they suffer?"[7]
Peter Singer, a bioethicist and author of Animal Liberation published in 1975, suggested that consciousness is not necessarily the key issue: just because animals have smaller brains, or are ‘less conscious’, this does not mean that they are not capable of feeling pain.
Bernard Rollin, the principal author of two U.S. federal laws regulating pain relief for animals, writes that researchers remained unsure into the 1980s as to whether animals experience pain.[8] In his interactions with scientists and other veterinarians, Rollin was regularly asked to "prove" that animals are conscious, and to provide "scientifically acceptable" grounds for claiming that they feel pain.[8]
Continuing into the 1990s, discussions were further developed on the roles that philosophy and science had in understanding animal cognition and mentality.[9] In subsequent years, it was argued there was strong support for the suggestion that some animals (most likely amniotes) have at least simple conscious thoughts and feelings[10] and that the view animals feel pain differently to higher primates is now a minority view.[4]
Scientific investigation
In the 20th- and 21st-century, there were many scientific investigations of pain in non-human animals.
Mammals
At the turn of the century, studies were published showing that arthritic rats self-select analgesic opiates.[12] In 2014, the veterinary Journal of Small Animal Practice published an article on the recognition of pain which started – "The ability to experience pain is universally shared by all mammals..."[13] and in 2015, it was reported in the science journal Pain, that several mammalian species (rat, mouse, rabbit, cat and horse) adopt a facial expression in response to a noxious stimulus that is consistent with the expression of pain.[14]
Birds
At the same time as the investigations using arthritic rats, studies were published showing that birds with gait abnormalities self-select for a diet that contains carprofen, an analgesic.[15] In 2005, it was written "Avian pain is likely analogous to pain experienced by most mammals"[16] and in 2014, "...it is accepted that birds perceive and respond to noxious stimuli and that birds feel pain."[17]
Reptiles
Veterinary articles have been published stating reptiles[18][19][20] experience pain in a way analogous to mammals, and that analgesics are effective in this class of vertebrates.
Fish
Several scientists or scientific groups have made statements indicating they believe fish can experience pain. For example, in 2004, Chandroo et al. wrote "Anatomical, pharmacological and behavioural data suggest that affective states of pain, fear and stress are likely to be experienced by fish in similar ways as in tetrapods".[21] In 2009, the European Food Safety Authority published a document stating scientific opinion on the welfare of fish. The document contains many sections indicating that the scientific panel believe fish can experience pain, for example, "Fish that are simply immobilized or paralysed [before euthanasia] would experience pain and suffering..."[22] In 2015, Brown wrote "A review of the evidence for pain perception strongly suggests that fish experience pain in a manner similar to the rest of the vertebrates."[23]
Argument by analogy
In 2012 the American philosopher Gary Varner reviewed the research literature on pain in animals. His findings are summarised in the following table.[24]
| Argument by analogy[24] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Property | |||||||||
| Fish | Amphibians | Reptiles | Birds | Mammals | |||||
| Has nociceptors | |||||||||
| Has brain | |||||||||
| Nociceptors and brain linked | ?[lower-alpha 1] / |
?[lower-alpha 2] / |
? / |
||||||
| Has endogenous opioids | |||||||||
| Analgesics affect responses | ?[lower-alpha 3] | ?[lower-alpha 4] | |||||||
| Response to damaging stimuli similar to humans | |||||||||
Notes
Arguing by analogy, Varner claims that any animal which exhibits the properties listed in the table could be said to experience pain. On that basis, he concludes that all vertebrates, including amphibians, probably experience pain, but invertebrates apart from cephalopods probably do not experience pain.[24][29]
Experiencing pain
Although there are numerous definitions of pain, almost all involve two key components.
First, nociception is required.[30] This is the ability to detect noxious stimuli which evoke a reflex response that rapidly moves the entire animal, or the affected part of its body, away from the source of the stimulus. The concept of nociception does not imply any adverse, subjective "feeling" – it is a reflex action. An example would be the rapid withdrawal of a finger that has touched something hot – the withdrawal occurs before any sensation of pain is actually experienced.
The second component is the experience of "pain" itself, or suffering – the internal, emotional interpretation of the nociceptive experience. This is when the withdrawn finger begins to hurt, moments after the withdrawal. Pain is therefore a private, emotional experience. Pain cannot be directly measured in other animals; responses to putatively painful stimuli can be measured, but not the experience itself. To address this problem when assessing the capacity of other species to experience pain, argument-by-analogy is used. This is based on the principle that if an animal responds to a stimulus in a similar way, it is likely to have had an analogous experience.
Nociception
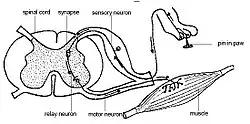
Nociception usually involves the transmission of a signal along a chain of nerve fibers from the site of a noxious stimulus at the periphery to the spinal cord and brain. This process evokes a reflex arc response generated at the spinal cord and not involving the brain, such as flinching or withdrawal of a limb. Nociception is found, in one form or another, across all major animal taxa.[30] Nociception can be observed using modern imaging techniques; and a physiological and behavioral response to nociception can be detected.
Emotional pain
Sometimes a distinction is made between "physical pain" and "emotional" or "psychological pain". Emotional pain is the pain experienced in the absence of physical trauma, e.g. the pain experienced after the loss of a loved one, or the break-up of a relationship. It has been argued that only primates can feel "emotional pain", because they are the only animals that have a neocortex – a part of the brain's cortex considered to be the "thinking area". However, research has provided evidence that monkeys, dogs, cats and birds can show signs of emotional pain and display behaviours associated with depression during painful experience, i.e. lack of motivation, lethargy, anorexia, unresponsiveness to other animals.[31]
Physical pain
The nerve impulses of the nociception response may be conducted to the brain thereby registering the location, intensity, quality and unpleasantness of the stimulus. This subjective component of pain involves conscious awareness of both the sensation and the unpleasantness (the aversive, negative affect). The brain processes underlying conscious awareness of the unpleasantness (suffering), are not well understood.
There have been several published lists of criteria for establishing whether non-human animals experience pain, e.g.[32][33] Some criteria that may indicate the potential of another species, including amphibians, to feel pain include:[33]
- Has a suitable nervous system and sensory receptors
- Has opioid receptors and shows reduced responses to noxious stimuli when given analgesics and local anaesthetics
- Physiological changes to noxious stimuli
- Displays protective motor reactions that might include reduced use of an affected area such as limping, rubbing, holding or autotomy
- Shows avoidance learning
- Shows trade-offs between noxious stimulus avoidance and other motivational requirements
- High cognitive ability and sentience
Adaptive value
The adaptive value of nociception is obvious; an organism detecting a noxious stimulus immediately withdraws the limb, appendage or entire body from the noxious stimulus and thereby avoids further (potential) injury. However, a characteristic of pain (in mammals at least) is that pain can result in hyperalgesia (a heightened sensitivity to noxious stimuli) and allodynia (a heightened sensitivity to non-noxious stimuli). When this heightened sensitisation occurs, the adaptive value is less clear. First, the pain arising from the heightened sensitisation can be disproportionate to the actual tissue damage caused. Second, the heightened sensitisation may also become chronic, persisting well beyond the tissues healing. This can mean that rather than the actual tissue damage causing pain, it is the pain due to the heightened sensitisation that becomes the concern. This means the sensitisation process is sometimes termed maladaptive. It is often suggested hyperalgesia and allodynia assist organisms to protect themselves during healing, but experimental evidence to support this has been lacking.[34][35]
In 2014, the adaptive value of sensitisation due to injury was tested using the predatory interactions between longfin inshore squid (Doryteuthis pealeii) and black sea bass (Centropristis striata) which are natural predators of this squid. If injured squid are targeted by a bass, they began their defensive behaviours sooner (indicated by greater alert distances and longer flight initiation distances) than uninjured squid. If anaesthetic (1% ethanol and MgCl2) is administered prior to the injury, this prevents the sensitisation and blocks the behavioural effect. The authors claim this study is the first experimental evidence to support the argument that nociceptive sensitisation is actually an adaptive response to injuries.[36]
Research findings
Receptors
Frogs have nociceptors in the superficial and deep layers of the skin that transduce mechanical and chemical noxious stimuli. Furthermore, frogs possess neural pathways that support processing and perception of noxious stimuli. Although organization is less well structured compared with mammals, it is now commonly accepted that amphibians possess neuro-anatomical pathways conductive of a complete nociceptive experience.[25]
Nerve fibres
Early electrophysiological studies in frogs report that noxious mechanical, thermal and chemical stimuli excite primary afferent fibres with slowly conducting axons.[37]
There are two types of nerve fibre relevant to pain in amphibians. Group C nerve fibres are a type of sensory nerve fibre which lack a myelin sheath and have a small diameter, meaning they have a low nerve conduction velocity. The suffering associated with burns, toothaches, or crushing injury are caused by C fibre activity. A-delta fibres are another type of sensory nerve fibre, however, these are myelinated and therefore transmit impulses faster than non-myelinated C fibres. A-delta fibres carry cold, pressure and some pain signals, and are associated with acute pain that results in "pulling away" from noxious stimuli.[38]
The skin of frogs contains both Group C fibres and A-delta fibres.[25][37]
Brain
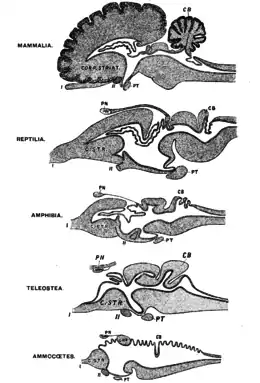
_(20356465586).jpg.webp)
All vertebrate species have a common brain archetype divided into the telencephalon and diencephalon (collectively referred to as forebrain), mesencephalon (midbrain) and rhombencephalon (hindbrain).[39] Nervous connections to the telencephalon indicate that frogs may be able to perceive pain.[25]
In 2002, James Rose, from the University of Wyoming, published reviews arguing that fish cannot feel pain because they lack a neocortex in the brain.[40][41] If the presence of a large, considerably developed neocortex is required for experiencing pain, as Rose suggests, this theory would eliminate birds, amphibians, other non-mammalian animals, and even some mammals from having the capacity to experience pain.[42] Other researchers do not believe that animal consciousness requires a neocortex, but can arise from homologous subcortical brain networks.[11] Animal behaviouralist Temple Grandin argues that fish (and therefore, presumably, amphibians) could still have consciousness without a neocortex because "different species can use different brain structures and systems to handle the same functions."[43]
Opioid system and effects of analgesics
By spinal administration of a range of opioid agonists, it has been demonstrated that frogs have mu (μ)-, delta (δ) and kappa (κ)-opioid binding sites.[44] The kappa sub-types κ1 and κ2 are present in the brains of edible frogs (Rana esculenta). In evolutionary terms, this means the opioid receptor sub-types are already present in amphibians, although the differences between these are less pronounced than in mammals.[45] Sequence comparisons show that the amphibian opioid receptors are highly conserved (70-84% similar to mammals) and are expressed in the central nervous system (CNS) areas apparently involved in pain experience.[32]
When treating amphibians, veterinary practice frequently uses the same analgesics and anesthetics used for mammals. These chemicals act on the nociceptive pathways, blocking signals to the brain where emotional responses to the signals are further processed by certain parts of the brain found in amniotes ("higher vertebrates").[46][47]
Effects of morphine and other opioids
The relative analgesic potency of 11 opioid agents (μ-opioid receptor agonists – fentanyl, levorphanol, methadone, morphine, meperidine and codeine; the partial μ agonist – buprenorphine; and the κ-opioid receptor agonists – nalorphine, bremazocine, U50488 and CI-977) in the Northern grass frog produced a dose-dependent and long-lasting analgesia which persists for at least 4 hours. The relative analgesic potency of μ-opioids in amphibians was correlated with the relative analgesic potency of these same agents recorded in on the mouse writhing and hot plate tests.[48][49] Other opioid analgesics are effective in amphibians, for example, butorphanol.[50]
Alfaxalone–butorphanol and alfaxalone–morphine combinations are comparable in terms of onset and duration of anaesthesia in Oriental fire-bellied toads (Bombina orientalis).[51]
When an isolated peptide termed "frog's nociception-related peptide" (fNRP) is injected into newts, it increases the latency for newts to flick their tails in response to a hot-beam. The effect is blocked by simultaneous injection of naloxone, thereby indicating evidence for the interaction of fNRP and opioid steps in the analgesia pathways of newts.[52]
Effects of opioid antagonists
Naloxone and naltrexone are both μ-opioid receptor antagonists which, in mammals, negate the analgesic effects of opioids. Morphine analgesia in frogs is blocked by both naloxone and naltrexone, indicating that the effect is mediated at least partially by opioid receptors.[53]
Effects of other analgesics
Direct intraspinal injection of the catecholamines epinephrine and norepinephrine, and the α-adrenergic agents dexmedetomidine and clonidine, produce a dose-dependent elevation of pain thresholds in the Northern leopard frog (Rana pipiens). This analgesia occurs without accompanying motor or sedative effects.[54]
A range of non-opioid drugs administered through the dorsal lymph sac of Northern leopard frogs has demonstrable analgesic effects, established by using the acetic acid test. Chlorpromazine and haloperidol (antipsychotics), chlordiazepoxide (a benzodiazepine) and diphenhydramine (a histamine antagonist) produced moderate to strong analgesic effects, whereas indomethacin and ketorolac (NSAIDs), and pentobarbital (a barbiturate) produced weaker analgesic effects.[55]
Physiological changes
In multiple animal studies, it has been shown that stress causes increases in glucocorticoid levels).[56] Frogs release corticosteroids in response to many environmental factors[57] and this pattern of release is often species-specific within Amphibia[58] More specifically, increased stocking density and hypoxia cause changes in cortisol (one of the glucocorticoids) and white blood cells in American bullfrog tadpoles (Lithobates catesbeianus) indicative of stress.[58]
Analgesia in amphibians can be measured using heart rate and respiratory rate.[51]
Protective motor responses
Amphibians exhibit classic wiping and withdrawal protective motor responses to noxious chemical, heat and mechanical stimuli.[32]
Acetic acid (a strong irritant) applied to the hindlimb of frogs elicits vigorous wiping of the exposed skin; both pH and osmolarity may contribute to the nociception produced.[59] This response is used in a standard test for analgesic effects in frogs, commonly termed the "acetic acid test". In this procedure, dilutions of the acid are placed drop-wise on the dorsum of the frog's thigh until the frog wipes the affected area.[55]
Newts flick their tails in response to it being irradiated by a hot beam,[52] in a very similar manner to that observed in rodents being used in the tail flick test.
The threshold to Von Frey hairs and response to nociceptive withdrawal can be used to measure the effectiveness of analgesia.[51]
Avoidance learning
Early studies showed that African clawed frogs (Xenopus laevis) learn to avoid electric shocks in an aquatic shuttle-box test[60] and similarly, cane toads (Bufo marinus) learn to avoid electric shocks in a T-maze.[61] Furthermore, American bullfrogs (Rana catesbiana) learn to inhibit their high-priority, biologically adaptive righting reflex to avoid electric shock; after training, they remain passively on their backs rather than exhibiting the normal short-latency, righting response.[62]
Batrachochytrium dendrobatidis is a chytrid fungus that causes the disease chytridiomycosis in amphibians; frogs learn to avoid the fungus after just one exposure.[63]
Trade-offs in motivation
A painful experience may change the motivation for normal behavioural responses. American bullfrogs learn to inhibit their high-priority, biologically adaptive righting reflex to avoid electric shock. After repeated exposure, they remain passively on their backs rather than exhibiting the normal, short-latency, righting response,[62] thereby showing a trade-off in motivation.
Cognitive ability and sentience

It has been argued that although a high cognitive capacity may indicate a greater likelihood of experiencing pain, it also gives these animals a greater ability to deal with this, leaving animals with a lower cognitive ability a greater problem in coping with pain.[64]
Habituation
Habituation is one of the simplest forms of animal learning. It has been stated there are no qualitative or quantitative differences between vertebrate species in this form of learning[65] indicating there is no difference between mammals and amphibians in this process.
Associative learning
Newts are capable of associative learning. They are able to associate chemical signals from a novel predator with another chemical stimulus when the second stimulus is the skin extract of another newt.[66]
Numeracy
At least some amphibians are capable of numeracy.[67][68] When offered live fruit flies (Drosophila virilis), salamanders choose the larger of 1 vs 2 and 2 vs 3. Frogs are able to distinguish between low numbers (1 vs 2, 2 vs 3, but not 3 vs 4) and large numbers (3 vs 6, 4 vs 8, but not 4 vs 6) of prey. This is irrespective of other characteristics, i.e. surface area, volume, weight and movement, although discrimination among large numbers may be based on surface area.[69]
Spatial orientation

The Rocky Mountain toad (Bufo woodhousii woodhousii) and Gulf Coast toad (Bufo valliceps) are able to discriminate between left and right positions in a T-maze.[70]
Both the terrestrial toad Rhinella arenarum[71] and the spotted salamander (Ambystoma maculatum)[72] can learn to orient in an open space using visual cues to get to a reward. Furthermore, they prefer using cues close to the reward. This shows a learning phenomenon previously recorded in other taxa including mammals, birds, fish and invertebrates.[71] It has been suggested that male dart frogs of the species Allobates femoralis use spatial learning for way-finding in their local area; they are able to find their way back to their territory when displaced several hundred metres, so long as they are displaced in their local area.[73]
Social learning
Wood frog (Rana sylvatica) tadpoles use social learning to acquire information about predators; the ratio of tutors to observers, but not group size, influences the intensity of learned predator recognition.[74] Wood frog tadpoles also exhibit local enhancement in their social learning, however, spotted salamander larvae do not; this difference in social learning could be largely due to differences in aquatic ecology between tadpoles and salamander larvae.[75]
Criteria for pain perception
Scientists have also proposed that in conjunction with argument-by-analogy, criteria of physiology or behavioural responses can be used to assess the possibility of non-human animals perceiving pain. The following is a table of criteria suggested by Sneddon et al.[32]
| Criteria for pain perception in amphibians | ||||
|---|---|---|---|---|
| Criteria | ||||
| Anura
|
Caudata
|
Gymnophiona
| ||
| Has nociceptors | ? | ? | ||
| Pathways to central nervous system | ? | ? | ||
| Central processing in brain | ? | ? | ||
| Receptors for analgesic drugs | ? | ? | ||
| Physiological responses | ? | ? | ||
| Movement away from noxious stimuli | ? | ? | ||
| Behavioural changes from norm | ? | ? | ||
| Protective behaviour | ? | ? | ||
| Responses reduced by analgesic drugs | ? | ? | ||
| Self-administration of analgesia | ? | ? | ? | |
| Responses with high priority over other stimuli | ? | ? | ||
| Pay cost to access analgesia | ? | ? | ? | |
| Altered behavioural choices/preferences | ? | ? | ||
| Relief learning | ? | ? | ? | |
| Rubbing, limping or guarding | ? | ? | ||
| Paying a cost to avoid noxious stimulus | ? | ? | ? | |
| Tradeoffs with other requirements | ? | ? | ||
Scientific statements
Several scientists have made statements indicating they believe amphibians can experience pain. For example, -
After examining the morphology of the nervous system of vertebrates, Somme concluded "...most four-legged vertebrates have some state of consciousness..."[76]
Gentz, in a paper on the surgery of amphibians, writes "Postoperative recommendations include ...analgesia" and "Hypothermia is also unacceptable as a sedation technique for painful procedures".[50]
Veterinary articles have been published stating amphibians experience pain in a way analogous to mammals, and that analgesics are effective in control of this class of vertebrates.[77][78][79] Shine et al., wrote that most animal ethics committees and the wider community believe that amphibians can feel pain.[80]
Some scientists have been a little more guarded about the experience of amphibians, for example, Michaels et al. wrote that the identification of pain pathways shared between amphibians and other amniotes suggests an ability to experience pain, even if in a different and more restricted sense than in amniote taxa.[81]
Societal implications
Societal implications of pain in amphibians include acute and chronic exposure to pollutants, cuisine and scientific research (e.g. genetic-modification may have detrimental effects on welfare, deliberately-imposed adverse physical, physiological and behavioural states, toe-clipping or other methods of invasive marking and handling procedures which may cause injury).
Culinary

It has been claimed that frogs killed for eating are "...sliced through the belly while they are still fully conscious and they can take up to an hour to die."[82]
Legislation
In the UK, the legislation protecting animals during scientific research, the "Animals (Scientific Procedures) Act 1986", protects amphibians from the moment they become capable of independent feeding.[83] The legislation protecting animals in most other circumstances in the UK is "The Animal Welfare Act, 2006" which states that in the Act, " “animal” means a vertebrate other than man",[84] thereby including amphibians.
The 1974 Norwegian Animal Rights Law states it relates to mammals, birds, frogs, salamanders, reptiles, fish, and crustaceans.[85]
In the US, the legislation protecting animals during scientific research is "The Animal Welfare Act".[86] This Act excludes protection of "cold-blooded" animals, thereby excluding amphibians from protection.
See also
References
- Broom, D.M. (2001). "Evolution of pain" (PDF). Vlaams Diergeneeskundig Tijdschrift. 70 (1): 17–21.
- Abbott, F.V., Franklin, K.B.J. and Westbrook, R.F. (1995). "The formalin test: Scoring properties of the first and second phases of the pain response in rats". Pain. 60 (1): 91–102. doi:10.1016/0304-3959(94)00095-V. PMID 7715946. S2CID 35448280.CS1 maint: multiple names: authors list (link)
- Key, B. (2015). "Fish do not feel pain and its implications for understanding phenomenal consciousness". Biology and Philosophy. 30 (2): 149–165. doi:10.1007/s10539-014-9469-4. PMC 4356734. PMID 25798021.
- Carbone, L. (2004). What Animals Want: Expertise and Advocacy in Laboratory Animal Welfare Policy. Oxford University Press. p. 149. ISBN 9780195161960.
- Radner, D. & Radner, M. (1989). Animal Consciousness. Prometheus Books: Buffalo.
- Harrison, P. (1992). "Descartes on animals". The Philosophical Quarterly. 42 (167): 219–227. doi:10.2307/2220217. JSTOR 2220217.
- "Bentham, J. (1879). An Introduction to the Principles of Morals and Legislation. Clarendon Press.
- Rollin, B. (1989). The Unheeded Cry: Animal Consciousness, Animal Pain, and Science. Oxford University Press, pp. xii, 117-118, cited in Carbone 2004, p. 150.
- Allen, C. (1998). "Assessing animal cognition: Ethological and philosophical perspectives". Journal of Animal Science. 76 (1): 42–47. doi:10.2527/1998.76142x. PMID 9464883.
- Griffin, D.R. & Speck, G.B. (2004). "New evidence of animal consciousness". Animal Cognition. 7 (1): 5–18. doi:10.1007/s10071-003-0203-x. PMID 14658059. S2CID 8650837.
- Low, P. (July 7, 2012). Jaak Panksepp; Diana Reiss; David Edelman; Bruno Van Swinderen; Philip Low; Christof Koch (eds.). "The Cambridge declaration on consciousness" (PDF). University of Cambridge.
- Colpaert, F.C., Tarayre, J.P., Alliaga, M., Slot. L.A.B., Attal, N. and Koek, W. (2001). "Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats". Pain. 91 (1–2): 33–45. doi:10.1016/s0304-3959(00)00413-9. PMID 11240076. S2CID 24858615.CS1 maint: multiple names: authors list (link)
- Mathews, K., Kronen, P.W., Lascelles, D., Nolan, A., Robertson, S., Steagall, P.V., Wright, B. and Yamashita, K. (2014). "Guidelines for recognition, assessment and treatment of pain". Journal of Small Animal Practice. 55 (6): E10–E68. doi:10.1111/jsap.12200. PMID 24841489.CS1 maint: multiple names: authors list (link)
- Chambers, C.T. and Mogil, J.S. (2015). "Ontogeny and phylogeny of facial expression of pain". Pain. 156 (5): 798–799. doi:10.1097/j.pain.0000000000000133. PMID 25887392. S2CID 2060896.CS1 maint: multiple names: authors list (link)
- Danbury, T.C., Weeks, C.A., Chambers, J.P., Waterman-Pearson, A.E. and Kestin, S.C. (2000). "Self-selection of the analgesic drug carprofen by lame broiler chickens". The Veterinary Record. 146 (11): 307–311. doi:10.1136/vr.146.11.307. PMID 10766114. S2CID 35062797.CS1 maint: multiple names: authors list (link)
- Machin, K.L. (2005). "Avian analgesia". Seminars in Avian and Exotic Pet Medicine. 14 (4): 236–242. doi:10.1053/j.saep.2005.09.004.
- Paul-Murphy, J. & Hawkins, M.G. (2014). "Chapter 26 - Bird-specific considerations: recognizing pain in pet birds.". In Gaynor, J.S. & Muir III, W. W. (eds.). Handbook of Veterinary Pain Management. Elsevier Health Sciences.
- Mosley, C.A. (2005). "Anesthesia & Analgesia in reptiles". Seminars in Avian and Exotic Pet Medicine. 14 (4): 243–262. doi:10.1053/j.saep.2005.09.005.
- Mosley, C. (2011). "Pain and nociception in reptiles". Veterinary Clinics of North America: Exotic Animal Practice. 14 (1): 45–60. doi:10.1016/j.cvex.2010.09.009. PMID 21074702.
- Sladky, K.K. & Mans, C. (2012). "Clinical analgesia in reptiles". Journal of Exotic Pet Medicine. 21 (2): 158–167. doi:10.1053/j.jepm.2012.02.012.
- Chandroo, K.P., Duncan, I.J. and Moccia, R.D. (2004). "Can fish suffer?: perspectives on sentience, pain, fear and stress". Applied Animal Behaviour Science. 86 (3): 225–250. CiteSeerX 10.1.1.327.8769. doi:10.1016/j.applanim.2004.02.004.CS1 maint: multiple names: authors list (link)
- Salman, J., Vannier, P. and Wierup. M. (2009). "Species-specific welfare aspects of the main systems of stunning and killing of farmed Atlantic salmon" (PDF). The EFSA Journal. 2012: 1–77.CS1 maint: multiple names: authors list (link)
- Brown, C. (2015). "Fish intelligence, sentience and ethics". Animal Cognition. 18 (1): 1–17. doi:10.1007/s10071-014-0761-0. PMID 24942105. S2CID 207050888.
- Varner, G.E. (2012). "Chapter 5 - Which animals are sentient? - the table in the article is based on Table 5.2, page 113". Personhood, Ethics, and Animal Cognition: Situating Animals in Hare's Two Level Utilitarianism. Oxford University Press. doi:10.1093/acprof:oso/9780199758784.001.0001. ISBN 9780199758784.
- Guénette, S.A., Giroux, M.C. and Vachon, P. (2013). "Pain perception and anaesthesia in research frogs". Experimental Animals. 62 (2): 87–92. doi:10.1538/expanim.62.87. PMID 23615302.CS1 maint: multiple names: authors list (link)
- Mosley, C. (2006). "Pain, nociception and analgesia in reptiles: when your snake goes 'ouch!'" (PDF). The North American Veterinary Conference. 20: 1652–1653.
- Coble, D.J., Taylor, D.K. and Mook, D.M. (2011). "Analgesic effects of meloxicam, morphine sulfate, flunixin meglumine, and xylazine hydrochloride in African-clawed frogs (Xenopus laevis)". Journal of the American Association for Laboratory Animal Science. 50 (3): 355–60. PMC 3103286. PMID 21640031.CS1 maint: multiple names: authors list (link)
- Baker, B.B., Sladky, K.K. and Johnson, S.M. (2011). "Evaluation of the analgesic effects of oral and subcutaneous tramadol administration in red-eared slider turtles". Journal of the American Veterinary Medical Association. 238 (2): 220–227. doi:10.2460/javma.238.2.220. PMC 3158493. PMID 21235376.CS1 maint: multiple names: authors list (link)
- Andrews, K. (2014). The Animal Mind: An Introduction to the Philosophy of Animal Cognition - see section 3.6.2. Routledge. ISBN 9781317676751.
- Sneddon, L.U. (2004). "Evolution of nociception in vertebrates: comparative analysis of lower vertebrates". Brain Research Reviews. 46 (2): 123–130. doi:10.1016/j.brainresrev.2004.07.007. PMID 15464201. S2CID 16056461.
- Sneddon, L.U. "Can animals feel pain?". The Welcome Trust. Archived from the original on April 13, 2012. Retrieved September 24, 2015.
- Sneddon, L.U., Elwood, R.W., Adamo, S.A. and Leach, M.C. (2014). "Defining and assessing animal pain". Animal Behaviour. 97: 201–212. doi:10.1016/j.anbehav.2014.09.007. S2CID 53194458.CS1 maint: multiple names: authors list (link)
- Elwood, R.W., Barr, S. and Patterson, L. (2009). "Pain and stress in crustaceans?". Applied Animal Behaviour Science. 118 (3): 128–136. doi:10.1016/j.applanim.2009.02.018.CS1 maint: multiple names: authors list (link)
- Price, T.J. & Dussor, G. (2014). "Evolution: the advantage of 'maladaptive'pain plasticity". Current Biology. 24 (10): R384–R386. doi:10.1016/j.cub.2014.04.011. PMC 4295114. PMID 24845663.
- "Maladaptive pain". Oxford Reference. Retrieved May 16, 2016.
- Crook, R.J., Dickson, K., Hanlon, R.T. and Walters, E.T. (2014). "Nociceptive sensitization reduces predation risk". Current Biology. 24 (10): 1121–1125. doi:10.1016/j.cub.2014.03.043. PMID 24814149.CS1 maint: multiple names: authors list (link)
- Hamamoto, D. T. & Simone, D.A. (2003). "Characterization of cutaneous primary afferent fibers excited by acetic acid in a model of nociception in frogs". Journal of Neurophysiology. 90 (2): 566–577. doi:10.1152/jn.00324.2003. PMID 12750420. S2CID 15575676.
- Rose, J.D., Arlinghaus, R., Cooke, S.J., Diggles, B.K., Sawynok, W., Stevens, E.D. and Wynne, C.D.L. (2012). "Can fish really feel pain?" (PDF). Fish and Fisheries. 15 (1): 97–133. doi:10.1111/faf.12010.CS1 maint: multiple names: authors list (link)
- Fabbro, F., Aglioti, S.M., Bergamasco, M., Clarici, A. and Panksepp, J. (2015). "Evolutionary aspects of self-and world consciousness in vertebrates". Frontiers in Human Neuroscience. 9: 157. doi:10.3389/fnhum.2015.00157. PMC 4374625. PMID 25859205.CS1 maint: multiple names: authors list (link)
- Rose, J.D. (2002). "The neurobehavioral nature of fishes and the question of awareness and pain" (PDF). Reviews in Fisheries Science. 10 (1): 1–38. CiteSeerX 10.1.1.598.8119. doi:10.1080/20026491051668. S2CID 16220451. Archived from the original (PDF) on 2012-10-10.
- Rose, J.D. (2002). "Do fish feel pain?". Archived from the original on January 20, 2013. Retrieved September 27, 2007.
- Yue, S. (2008). "An HSUS report: fish and pain perception". Impacts on Farm Animals. Retrieved October 21, 2015.
- Grandin, T. & Johnson, C. (2005). Animals in Translation. New York: Scribner. pp. 183–184. ISBN 978-0-7432-4769-6.
- Stevens, C.W. (1996). "Relative analgesic potency of mu, delta and kappa opioids after spinal administration in amphibians". Journal of Pharmacology and Experimental Therapeutics. 276 (2): 440–448.
- Benyhe, S., Varga, E., Hepp, J., Magyar, A., Borsodi, A. and Wollemann, M. (1990). "Characterization of kappa1 and kappa2 opioid binding sites in frog (Rana esculenta) brain membrane preparation". Neurochemical Research. 15 (9): 899–904. doi:10.1007/bf00965909. PMID 2177154. S2CID 23867820.CS1 maint: multiple names: authors list (link)
- Viñuela-Fernández I, Jones E, Welsh EM, Fleetwood-Walker SM (September 2007). "Pain mechanisms and their implication for the management of pain in farm and companion animals". Vet. J. 174 (2): 227–39. doi:10.1016/j.tvjl.2007.02.002. PMID 17553712.
- Sneddon, L.U. (2012). "Clinical Anesthesia & Analgesia in fish". Journal of Exotic Pet Medicine. 21: 32–43. doi:10.1053/j.jepm.2011.11.009.
- Stevens, C.W., Klopp, A.J. and Facello, J.A. (1994). "Analgesic potency of mu and kappa opioids after systemic administration in amphibians". Journal of Pharmacology and Experimental Therapeutics. 269 (3): 1086–1093.CS1 maint: multiple names: authors list (link)
- Stevens, C.W., MacIver, D.N. and Newman, L.C. (2001). "Testing and comparison of non-opioid analgesics in amphibians". Contemporary Topics in Laboratory Animal Science. 40 (4): 23–7. PMC 3075466. PMID 11451391.CS1 maint: multiple names: authors list (link)
- Gentz E.J. (2007). "Medicine and surgery of amphibians". ILAR Journal. 48 (3): 255–259. doi:10.1093/ilar.48.3.255. PMID 17592187.
- Adami, C., Spadavecchia, C., Angeli, G. and d'Ovidio, D. (2015). "Alfaxalone anesthesia by immersion in oriental fire‐bellied toads (Bombina orientalis)". Veterinary Anaesthesia and Analgesia. 42 (5): 547–551. doi:10.1111/vaa.12252. PMID 25711769.CS1 maint: multiple names: authors list (link)
- Kanetoh, T., Sugikawa, T., Sasaki, I., Muneoka, Y., Minakata, H., Takabatake, I. and Fujimoto, M. (2003). "Identification of a novel frog RFamide and its effect on the latency of the tail-flick response of the newt". Comparative Biochemistry and Physiology C. 134 (2): 259–266. doi:10.1016/s1532-0456(02)00277-6. PMID 12600686.CS1 maint: multiple names: authors list (link)
- Suckow, M.A., Terril, L.A., Grigdesby, C.F. and March, P.A. (1999). "Evaluation of hypothermia-induced analgesia and influence of opioid antagonists in Leopard frogs (Rana pipiens)". Pharmacology Biochemistry and Behavior. 63 (1): 39–43. doi:10.1016/s0091-3057(98)00237-8. PMID 10340522. S2CID 22059545.CS1 maint: multiple names: authors list (link)
- Stevens, C.W. & Brenner, G.M. (1996). "Spinal administration of adrenergic agents produces analgesia in amphibians". European Journal of Pharmacology. 316 (2): 205–210. doi:10.1016/s0014-2999(96)00681-4. PMID 8982687.
- Stevens, C.W., MacIver, D.N. and Newman, L.C. (2001). "Testing and comparison of non-opioid analgesics in amphibians". Contemporary Topics in Laboratory Animal Science. 40 (4): 23–7. PMC 3075466. PMID 11451391.CS1 maint: multiple names: authors list (link)
- Carlson, N.R. (2010). Physiology of Behavior, 11th Edition. New York: Allyn & Bacon. p. 605.
- Hanke, W. (2013) [1978]. "Chapter 5. The adrenal cortex of Amphibia". In I. Chester Jones; I.W. Henderson (eds.). General, Comparative and Clinical Endocrinology of the Adrenal Cortex, Volume 2. Academic Press. pp. 419–487.
- Teixeira, P.C., Dias, D.C., Rocha, G.C., Antonucci, A.M., França, F.M., Marcantonio, A.S., Ranzani-Paiva, M.T. and Ferreira, C.M. (2012). "Profile of cortisol, glycaemia, and blood parameters of American Bullfrog tadpoles Lithobates catesbeianus exposed to density and hypoxia stressors". Pesquisa Veterinária Brasileira. 32: 91–98. doi:10.1590/s0100-736x2012001300016.CS1 maint: multiple names: authors list (link)
- Hamamoto, D.T., Forkey, M.W., Davis, W.L., Kajander, K.C. and Simone, D.A. (2000). "The role of pH and osmolarity in evoking the acetic acid-induced wiping response in a model of nociception in frogs". Brain Research. 862 (1): 217–229. doi:10.1016/s0006-8993(00)02138-7. PMID 10799688. S2CID 7290178.CS1 maint: multiple names: authors list (link)
- Miller, R.R., Berk, A.M. and Springer, A.D. (1974). "Acquisition and retention of active avoidance in Xenopus laevis". Bulletin of the Psychonomic Society. 3 (2): 139–141. doi:10.3758/BF03333423.CS1 maint: multiple names: authors list (link)
- Williams Jr, J.T. (1967). "A test for dominance of cues during maze learning by toads". Psychonomic Science. 9 (5): 259–260. doi:10.3758/bf03332211. S2CID 143932516.
- Harvey, C.B., Ellis, C. and Tate, M. (1976). "Inhibition of the righting reflex in the common bullfrog (Rana catesbiana) employing an operant-avoidance procedure". Bulletin of the Psychonomic Society. 7 (1): 57–58. doi:10.3758/bf03337120.CS1 maint: multiple names: authors list (link)
- McMahon, T.A., Sears, B.F., Venesky, M.D., Bessler, S.M., Brown, J.M., Deutsch, K., ... and Rohr, J.R. (2014). "Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression". Nature. 511 (7508): 224–227. Bibcode:2014Natur.511..224M. doi:10.1038/nature13491. PMC 4464781. PMID 25008531.CS1 maint: multiple names: authors list (link)
- Broom, D.M. (2001). "Evolution of pain" (PDF). Vlaams Diergeneeskundig Tijdschrift. 70 (1): 17–21.
- Lea, S.E.G. (1983). "Complex general learning in nonmammalian vertebrates.". In Marler, P.; Terrace, H.S (eds.). The Biology of Learning: Report of the Dahlem Workshop on the Biology of Learning, Berlin. Springer-Verlag. ISBN 9783642700941.
- Vitti, J. (2010). The Distribution and Evolution of Animal Consciousness (Doctoral dissertation, Harvard University)
- Krusche, P., Uller, C. and Dicke, U. (2010). "Quantity discrimination in salamanders". Journal of Experimental Biology. 213 (11): 1822–1828. doi:10.1242/jeb.039297. PMID 20472768.CS1 maint: multiple names: authors list (link)
- Uller, C., Jaeger, R. and Guidry, G. (2003). "Salamanders (Plethodon cinereus) go for more: rudiments of number in an amphibian". Animal Cognition. 6 (2): 105–112. doi:10.1007/s10071-003-0167-x. PMID 12709845. S2CID 147018.CS1 maint: multiple names: authors list (link)
- Stancher, G., Rugani, R., Regolin, L. and Vallortigara, G. (2015). "Numerical discrimination by frogs (Bombina orientalis)". Animal Cognition. 18 (1): 219–229. doi:10.1007/s10071-014-0791-7. PMID 25108417. S2CID 16499583.CS1 maint: multiple names: authors list (link)
- Chu, P.K. & McCain, G. (1969). "Discrimination learning and extinction in toads". Psychonomic Science. 14 (1): 14–15. doi:10.3758/bf03336400.
- Daneri, M.F., Casanave, E.B. and Muzio, R.N. (2015). "Use of local visual cues for spatial orientation in terrestrial toads (Rhinella arenarum): The role of distance to a goal". Journal of Comparative Psychology. 129 (3): 247–255. doi:10.1037/a0039461. PMID 26147701. S2CID 30988123.CS1 maint: multiple names: authors list (link)
- Heuring, W. L. & Mathis, A. (2014). "Landmark learning by juvenile salamanders (Ambystoma maculatum)". Behavioural Processes. 108: 173–176. doi:10.1016/j.beproc.2014.10.015. PMID 25444775. S2CID 45373288.
- Pašukonis, A., Warrington, I., Ringler, M. and Hödl, W. (2014). "Poison frogs rely on experience to find the way home in the rainforest". Biology Letters. 10 (11): 20140642. doi:10.1098/rsbl.2014.0642. PMC 4261859. PMID 25411379.CS1 maint: multiple names: authors list (link)
- Chivers, D.P. & Ferrari, M.C. (2015). "The effect of group size and tutor-to-observer ratio on socially learned antipredator responses in woodfrog tadpoles". Animal Behaviour. 104: 25–29. doi:10.1016/j.anbehav.2015.03.003. S2CID 53165376.
- Chapman, T.L., Holcomb, M.P., Spivey, K.L., Sehr, E.K. and Gall, B.G. (2015). "A test of local enhancement in amphibians". Ethology. 121 (3): 308–314. doi:10.1111/eth.12337.CS1 maint: multiple names: authors list (link)
- Sømme, L.S. (2005). "Sentience and pain in invertebrates. Report to Norwegian Scientific Committee for Food Safety" (PDF). Norwegian University of Life Sciences. Cite journal requires
|journal=(help) - Machin, K.L. (1999). "Amphibian pain and analgesia". Journal of Zoo and Wildlife Medicine. 30 (1): 2–10. JSTOR 20095815. PMID 10367638.
- Machin, K.L. (2001). "Fish, amphibian, and reptile analgesia". The Veterinary Clinics of North America. Exotic Animal Practice. 4 (1): 19–33. doi:10.1016/S1094-9194(17)30048-8. PMID 11217460.
- Stevens, C.W. (2011). "Analgesia in amphibians: preclinical studies and clinical applications". Veterinary Clinics of North America: Exotic Animal Practice. 14 (1): 33–44. doi:10.1016/j.cvex.2010.09.007. PMC 3056481. PMID 21074701.
- Shine, R., Amiel, J., Munn, A.J., Stewart, M., Vyssotski, A.L., and Lesku, J.A. (2015). "Is "cooling then freezing" a humane way to kill amphibians and reptiles?". Biology Open. 4 (7): 760–763. doi:10.1242/bio.012179. PMC 4571096. PMID 26015533.CS1 maint: multiple names: authors list (link)
- Michaels, C.J.; Downie, J.R.; Campbell-Palmer, R. (2014). "The importance of enrichment for advancing amphibian welfare and conservation goals" (PDF). Amphibian & Reptile Conservation. 8 (1): 7–23. Archived from the original (PDF) on 2015-02-03.
- BBC news (2010). "Protest over 'cruel' frogs' legs chippy in Sunderland". BBC. Retrieved October 10, 2015.
- "Animals (Scientific Procedures) Act 1986" (PDF). Home Office (UK). Retrieved September 23, 2015.
- "Animal Welfare Act 2006". UK Government. 2006. Retrieved September 25, 2015.
- Henriksen, S., Vaagland, H., Sundt-Hansen, L., May, R. and Fjellheim, A. (2003). "Consequences of pain perception in fish for catch and release, aquaculture and commercial fisheries" (PDF).CS1 maint: multiple names: authors list (link)
- "Rules and regulations: Animal welfare act". New England Anti-Vivisection Society (NEAVS). Retrieved October 25, 2015.


.png.webp)