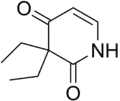
Pyrithyldione
Pyrithyldione (Presidon, Persedon) is a psychoactive drug invented in 1949.[1] An improved method of manufacture was patented by Roche in 1959.[2] It was used as a hypnotic or sedative and presumed to be less toxic than barbiturates.[3] Today, this substance is no longer used. Agranulocytosis was sometimes reported as adverse effect.[4][5]Pyrithyldione is also a CYP2D6 inducer but is not as potent as glutethimide[6] In studies, it increased the O-Demethylation of codeine by 20%.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Benedorm, Didropyridine, Dihydroprylone, Persedon, Presidon, Pyridion, Pyridione, Pyrithyldion, Pyrithyldione, Tetridin, Tetridine |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.910 |
| Chemical and physical data | |
| Formula | C9H13NO2 |
| Molar mass | 167.208 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
See also
References
- Becker EL, Fabing HD, Hawkins JR (April 1949). "Presidon; a new sedative-hypnotic". Christ Hospital Medical Bulletin. 2 (4): 80–4. PMID 18144514.
- US patent 3019230, "Method for the preparation of 2,4-dioxo-tetrahydropyridines", issued 1962-01-30, assigned to Hoffmann-La Roche
- Pribilla, O. (1956). "Zur Toxikologie des Persedons". Archiv für Toxikologie. 16 (1): 34–49. doi:10.1007/BF00577351. S2CID 38210598.
- Ibáñez L, Ballarín E, Pérez E, Vidal X, Capellà D, Laporte JR (January 2000). "Agranulocytosis induced by pyrithyldione, a sedative hypnotic drug". European Journal of Clinical Pharmacology. 55 (10): 761–4. doi:10.1007/s002280050011. PMID 10663456. S2CID 25595314.
- Covner AH, Halpern SL (January 1950). "Fatal agranulocytosis following therapy with presidon (3,3-diethyl-2,4-dioxotetrahydropyridine) a new sedative hypnotic agent". The New England Journal of Medicine. 242 (2): 49–52. doi:10.1056/NEJM195001122420203. PMID 15399031.
- [Is pyrithyldione (Benedorm) an enzyme inducer (author's transl)] Pharmazie. 1982 Jan;37(1):69.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.