Polyphenol
Polyphenols (/ˌpɒliˈfiːnoʊl, -nɒl/) are a large family of naturally occurring organic compounds characterized by multiples of phenol units.[1] They are abundant in plants and structurally diverse.[1][2][3] Polyphenols include flavonoids, tannic acid, and ellagitannin, some of which have been used historically as dyes and for tanning garments.
Definition of the term polyphenol
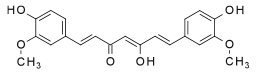
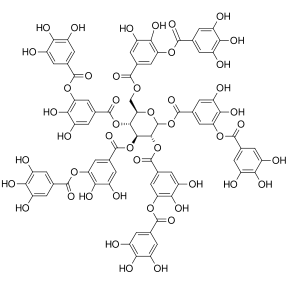
The term polyphenol is not well defined, but it is generally agreed that they are natural products "having a polyphenol structure (i.e., several hydroxyl groups on aromatic rings)" including four principal classes: "phenolic acids, flavonoids, stilbenes, and lignans".[4]
- Flavonoids include flavones, flavonols, flavanols, flavanones, isoflavones, proanthocyanidins, and anthocyanins. Particularly abundant flavanoids in foods are catechin (tea, fruits), hesperetin (citrus fruits), cyanidin (red fruits and berries), daidzein (soybean), proanthocyanidins (apple, grape, cocoa), and quercetin (onion, tea, apples).[2]
- Phenolic acid include caffeic acid
- Lignans are polyphenols derived from phenylalanine found in flax seed and other cereals.
"WBSSH" definition of polyphenols
The White–Bate-Smith–Swain–Haslam (WBSSH) definition[5] characterized structural characteristics common to plant phenolics used in tanning (i.e., the tannins).[6] In terms of properties, the WBSSH describes the polyphenols thusly:
- generally moderately water-soluble compounds
- with molecular weight of 500–4000 Da
- with >12 phenolic hydroxyl groups
- with 5–7 aromatic rings per 1000 Da
In terms of structures, the WBSSH recognizes two structural family that have these properties:
- proanthocyanidins and its derivatives
- galloyl and hexahydroxydiphenoyl esters and their derivatives
Quideau definition of polyphenols
According to Stéphane Quideau the term "polyphenol" refers to compounds derived from the shikimate/phenylpropanoid and/or the polyketide pathway, featuring more than one phenolic unit and deprived of nitrogen-based functions.
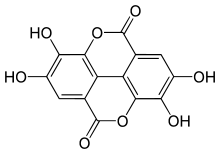
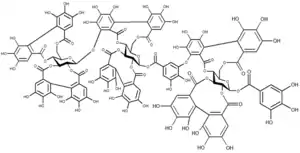
Ellagic acid (M.W. 302, right), a molecule at the core of naturally occurring phenolic compounds of varying sizes, is itself not a polyphenol by the WBSSH definition, but is by the Quideau definition. The raspberry ellagitannin (M.W. ~2450),[7] on the other hand, with its 14 gallic acid moieties (most in ellagic acid-type components), and more than 40 phenolic hydroxyl groups, meets the criteria of both definitions of a polyphenol. Other examples of compounds that fall under both the WBSSH and Quideau definitions include the black tea theaflavin-3-gallate shown below, and the hydrolyzable tannin, tannic acid, shown above.
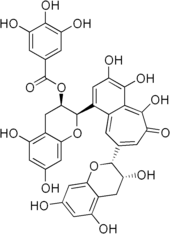
Structure and biosynthesis
Structural features
Polyphenols are often larger molecules (macromolecules). Their upper molecular weight limit is about 800 Daltons, which allows for the possibility to rapidly diffuse across cell membranes so that they can reach intracellular sites of action or remain as pigments once the cell senesces. Hence, many larger polyphenols are biosynthesized in-situ from smaller polyphenols to non-hydrolyzable tannins and remain undiscovered in the plant matrix. Most polyphenols contain repeating phenolic moieties of pyrocatechol, resorcinol, pyrogallol, and phloroglucinol connected by esters (hydrolyzable tannins) or more stable C-C bonds (nonhydrolyzable condensed tannins). Proanthocyanidins are mostly polymeric units of catechin and epicatechin.
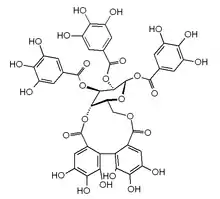
Polyphenols often have functional groups beyond hydroxyl groups. Ether ester linkages are common, as are carboxylic acids.
Chemical properties
Polyphenols are reactive species toward oxidation, hence their description as antioxidants in vitro.[8]
Uses
Some polyphenols are traditionally used as dyes. For instance, in the Indian subcontinent, the pomegranate peel, high in tannins and other polyphenols, or its juice, is employed in the dyeing of non-synthetic fabrics.[9]
Polyphenols, especially tannins, were used traditionally for tanning leather and today also as precursors in green chemistry[10] notably to produce plastics or resins by polymerisation with[11] or without the use of formaldehyde[12] or adhesives for particleboards.[13] The aims are generally to make use of plant residues from grape, olive (called pomaces) or pecan shells left after processing.[14]
Pyrogallol and pyrocatechin are among the oldest photographic developers.[15]
Biology
Polyphenols are thought to play diverse roles in the ecology of plants. These functions include:[16]
- Release and suppression of growth hormones such as auxin.
- UV screens to protect against ionizing radiation and to provide coloration (plant pigments).[4]
- Deterrence of herbivores (sensory properties).
- Prevention of microbial infections (phytoalexins).[4][17]
- Signaling molecules in ripening and other growth processes.
Occurrence in nature
The most abundant polyphenols are the condensed tannins, found in virtually all families of plants. Larger polyphenols are often concentrated in leaf tissue, the epidermis, bark layers, flowers and fruits but also play important roles in the decomposition of forest litter, and nutrient cycles in forest ecology. Absolute concentrations of total phenols in plant tissues differ widely depending on the literature source, type of polyphenols and assay; they are in the range of 1-25% total natural phenols and polyphenols, calculated with reference to the dry green leaf mass.[18]
High levels of polyphenols in some woods can explain their natural preservation against rot.[19]
Flax and Myriophyllum spicatum (a submerged aquatic plant) secrete polyphenols that are involved in allelopathic interactions.[20][21]
Polyphenols are also found in animals. In arthropods such as insects[22] and crustaceans[23] polyphenols play a role in epicuticle hardening (sclerotization). The hardening of the cuticle is due to the presence of a polyphenol oxidase.[24] In crustaceans, there is a second oxidase activity leading to cuticle pigmentation.[25] There is apparently no polyphenol tanning occurring in arachnids cuticle.[26]
Biosynthesis and metabolism
Polyphenols incorporate smaller parts and building blocks from simpler natural phenols, which originate from the phenyl propanoid pathway for the phenolic acids or the shikimic acid pathway for gallotannins and analogs. Flavonoids and caffeic acid derivatives are biosynthesized from phenyl alanine and malonyl-CoA. Complex gallotannins develop through the in-vitro oxidation of 1,2,3,4,6-pentagalloyl-glucose or dimerization processes resulting in hydrolyzable tannins. For anthocyanidins, precursors of the condensed tannin biosynthesis, dihydroflavonol reductase and leucoanthocyanidin reductase (LAR) are crucial enzymes with subsequent addition of catechin and epicatechin moieties for larger, non-hydrolyzable tannins.[27]
The glycosylated form develops from glucosyltransferase activity and increases the solubility of polyphenols.[28]
Polyphenol oxidase (PPO) is an enzyme that catalyses the oxidation of o-diphenols to produce o-quinones. It is the rapid polymerisation of o-quinones to produce black, brown or red polyphenolic pigments that is the cause of fruit browning. In insects, PPO serves for the cuticle hardening.[29]
Content in food
Polyphenols comprise up to 0.2–0.3% fresh weight for many fruits, grapes, and berries. Consuming common servings of wine, chocolate, legumes or tea may also contribute to about one gram of intake per day.[2][30] According to a 2005 review on polyphenols:
The most important food sources are commodities widely consumed in large quantities such as fruit and vegetables, green tea, black tea, red wine, coffee, chocolate, olives, and extra virgin olive oil. Herbs and spices, nuts and algae are also potentially significant for supplying certain polyphenols. Some polyphenols are specific to particular food (flavanones in citrus fruit, isoflavones in soya, phloridzin in apples); whereas others, such as quercetin, are found in all plant products such as fruit, vegetables, cereals, leguminous plants, tea, and wine.[31]
Some polyphenols are considered antinutrients – compounds that interfere with the absorption of essential nutrients – especially iron and other metal ions, which may bind to digestive enzymes and other proteins, particularly in ruminants.[32]
In a comparison of cooking methods, phenolic and carotenoid levels in vegetables were retained better by steaming compared to frying.[33] Polyphenols in wine, beer and various nonalcoholic juice beverages can be removed using finings, substances that are usually added at or near the completion of the processing of brewing.
Potential health effects
Although health effects may be attributed to polyphenols in food,[34] the extensive metabolism of polyphenols in the intestine and liver, and their undefined fate as metabolites which are rapidly excreted in urine, prevents definition of their biological effects.[2] Because the metabolism of polyphenols cannot be assessed in vivo, there are no Dietary Reference Intake (DRI) levels established or recommended.[2]
In the US, the Food and Drug Administration (FDA) issued labeling guidance to manufacturers that polyphenols cannot be mentioned as antioxidant nutrients unless physiological evidence exists to verify such a qualification and a DRI value has been established.[35][36] Furthermore, since purported health claims for specific polyphenol-enriched foods remain unproven,[37] health statements about polyphenols on product labels are prohibited by the FDA[36] and the EFSA.[38] However, during the 21st century, the EFSA recognized certain health claims of specific polyphenol products, such as cocoa[39] and olive oil.[40]
Compared with the effects of polyphenols in vitro, the possible functions in vivo remain unknown due to 1) the absence of validated in vivo biomarkers;[2] 2) long-term studies failing to demonstrate effects with a mechanism of action, sensitivity and specificity or efficacy;[2] and 3) invalid applications of high, unphysiological test concentrations in the in vitro studies, which are subsequently irrelevant for the design of in vivo experiments.[31]
Analysis techniques
Sensory properties
With respect to food and beverages, the cause of astringency is not fully understood, but it is measured chemically as the ability of a substance to precipitate proteins.[41]
A review published in 2005 found that astringency increases and bitterness decreases with the mean degree of polymerization. For water-soluble polyphenols, molecular weights between 500 and 3000 were reported to be required for protein precipitation. However, smaller molecules might still have astringent qualities likely due to the formation of unprecipitated complexes with proteins or cross-linking of proteins with simple phenols that have 1,2-dihydroxy or 1,2,3-trihydroxy groups.[42] Flavonoid configurations can also cause significant differences in sensory properties, e.g. epicatechin is more bitter and astringent than its chiral isomer catechin. In contrast, hydroxycinnamic acids do not have astringent qualities, but are bitter.[43]
Analysis
The analysis techniques are those of phytochemistry: extraction, isolation, structural elucidation,[44] then quantification.
Extraction
Extraction of polyphenols[45] can be performed using a solvent like water, hot water, methanol, methanol/formic acid, methanol/water/acetic or formic acid. Liquid–liquid extraction can be also performed or countercurrent chromatography. Solid phase extraction can also be made on C18 sorbent cartridges. Other techniques are ultrasonic extraction, heat reflux extraction, microwave-assisted extraction,[46] critical carbon dioxide,[14][47] pressurized liquid extraction[48] or use of ethanol in an immersion extractor.[49] The extraction conditions (temperature, extraction time, ratio of solvent to raw material, solvent and concentrations) have to be optimized.
Mainly found in the fruit skins and seeds, high levels of polyphenols may reflect only the measured extractable polyphenol (EPP) content of a fruit which may also contain non-extractable polyphenols. Black tea contains high amounts of polyphenol and makes up for 20% of its weight.[50]
Concentration can be made by ultrafiltration.[51] Purification can be achieved by preparative chromatography.
Analysis techniques
Phosphomolybdic acid is used as a reagent for staining phenolics in thin layer chromatography. Polyphenols can be studied by spectroscopy, especially in the ultraviolet domain, by fractionation or paper chromatography. They can also be analysed by chemical characterisation.
Instrumental chemistry analyses include separation by high performance liquid chromatography (HPLC), and especially by reversed-phase liquid chromatography (RPLC), can be coupled to mass spectrometry.[14] Purified compounds can be identified by the means of nuclear magnetic resonance.
Microscopy analysis
The DMACA reagent is an histological dye specific to polyphenols used in microscopy analyses. The autofluorescence of polyphenols can also be used, especially for localisation of lignin and suberin. Where fluorescence of the molecules themselves is insufficient for visualization by light microscopy, DPBA (diphenylboric acid 2-aminoethyl ester, also referred to as Naturstoff reagent A) has traditionally been used, at least in plant science, to enhance the fluorescence signal.[52]
Quantification
Polyphenolic content can be quantified separation/isolation by volumetric titration. An oxidizing agent, permanganate, is used to oxidize known concentrations of a standard tannin solution, producing a standard curve. The tannin content of the unknown is then expressed as equivalents of the appropriate hydrolyzable or condensed tannin.[53]
Some methods for quantification of total polyphenol content are based on colorimetric measurements. Some tests are relatively specific to polyphenols (for instance the Porter's assay). Total phenols (or antioxidant effect) can be measured using the Folin-Ciocalteu reaction.[14] Results are typically expressed as gallic acid equivalents. Polyphenols are seldom evaluated by antibody technologies.[54]
Other tests measure the antioxidant capacity of a fraction. Some make use of the ABTS radical cation which is reactive towards most antioxidants including phenolics, thiols and vitamin C.[55] During this reaction, the blue ABTS radical cation is converted back to its colorless neutral form. The reaction may be monitored spectrophotometrically. This assay is often referred to as the Trolox equivalent antioxidant capacity (TEAC) assay. The reactivity of the various antioxidants tested are compared to that of Trolox, which is a vitamin E analog.
Other antioxidant capacity assays which use Trolox as a standard include the diphenylpicrylhydrazyl (DPPH), oxygen radical absorbance capacity (ORAC),[56] ferric reducing ability of plasma (FRAP)[57] assays or inhibition of copper-catalyzed in vitro human low-density lipoprotein oxidation.[58]
New methods including the use of biosensors can help monitor the content of polyphenols in food.[59]
Quantitation results produced by the mean of diode array detector–coupled HPLC are generally given as relative rather than absolute values as there is a lack of commercially available standards for all polyphenolic molecules.
Etymology
The name derives from the Ancient Greek word πολύς (polus, meaning "many, much") and the word phenol which refers to a chemical structure formed by attaching to an aromatic benzenoid (phenyl) ring to a hydroxyl (-OH) group as is found in alcohols (hence the -ol suffix). The term polyphenol has been in use at least since 1894.[60]
See also
References
- Quideau, S. P.; Deffieux, D.; Douat-Casassus, C. L.; Pouységu, L. (2011). "Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis". Angewandte Chemie International Edition. 50 (3): 586–621. doi:10.1002/anie.201000044. PMID 21226137.
- "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 1 February 2016. Retrieved 28 October 2020.
- Nonaka, G. (1989). "Isolation and structure elucidation of tannins" (PDF). Pure Appl. Chem. 61 (3): 357–360. doi:10.1351/pac198961030357. S2CID 84226096.
- Manach, Claudine; Scalbert, Augustin; Morand, Christine; Rémésy, Christian; Jiménez, Liliana (1 May 2004). "Polyphenols: food sources and bioavailability". The American Journal of Clinical Nutrition. 79 (5): 727–747. doi:10.1093/ajcn/79.5.727. ISSN 0002-9165. PMID 15113710.
- Haslam, E.; Cai, Y. (1994). "Plant polyphenols (vegetable tannins): Gallic acid metabolism". Natural Product Reports. 11 (1): 41–66. doi:10.1039/NP9941100041. PMID 15206456.
- Practical Polyphenolics, Edwin Haslam, 1998, ISBN 0-521-46513-3
- Cardiovascular disease and phytochemicals. Anonymous. C. Hamilton et al.
- Santos, M.A; Bonilla Venceslada, J.L; Martin Martin, A; Garcia Garcia, I (2005). "Estimating the selectivity of ozone in the removal of polyphenols from vinasse". Journal of Chemical Technology and Biotechnology. 80 (4): 433–438. doi:10.1002/jctb.1222. INIST:16622840.
- K. K. Jindal; R. C. Sharma (2004). Recent trends in horticulture in the Himalayas. Indus Publishing. ISBN 978-81-7387-162-7.
... bark of tree and rind of fruit is commonly used in ayurveda ... also used for dyeing ...
- Polshettiwar, Vivek; Varma, Rajender S. (2008). "Greener and expeditious synthesis of bioactive heterocycles using microwave irradiation". Pure and Applied Chemistry. 80 (4): 777–790. doi:10.1351/pac200880040777. S2CID 11940026.
- Hillis, W. E.; Urbach, G. (1959). "Reaction of polyphenols with formaldehyde". Journal of Applied Chemistry. 9 (12): 665–673. doi:10.1002/jctb.5010091207.
- Fukuoka, Tokuma; Uyama, Hiroshi; Kobayashi, Shiro (2003). "Synthesis of Ultrahigh Molecular Weight Polyphenols by Oxidative Coupling". Macromolecules. 36 (22): 8213–8215. Bibcode:2003MaMol..36.8213F. doi:10.1021/ma034803t.
- Pizzi, A.; Valenezuela, J.; Westermeyer, C. (1994). "Low formaldehyde emission, fast pressing, pine and pecan tannin adhesives for exterior particleboard". Holz Als Roh- und Werkstoff. 52 (5): 311–315. doi:10.1007/BF02621421. S2CID 36500389.
- Aizpurua-Olaizola, Oier; Ormazabal, Markel; Vallejo, Asier; Olivares, Maitane; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (2015). "Optimization of Supercritical Fluid Consecutive Extractions of Fatty Acids and Polyphenols from Vitis Vinifera Grape Wastes". Journal of Food Science. 80 (1): E101–107. doi:10.1111/1750-3841.12715. PMID 25471637.
- Stephen G. Anchell & Bill Troop (1998). The Film Developing Cookbook. p. 25. ISBN 978-0240802770.
- V. Lattanzio et al. (2006). "Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects" (and references therein). Phytochemistry: Advances in Research, 23–67. ISBN 81-308-0034-9.
- Huber, B; Eberl, L; Feucht, W; Polster, J (2003). "Influence of polyphenols on bacterial biofilm formation and quorum-sensing". Z. Naturforsch. C. 58 (11–12): 879–884. doi:10.1515/znc-2003-11-1224. PMID 14713169. S2CID 25764128.
- Hättenschwiler, Stephan; Vitousek, Peter M (2000). "The role of polyphenols in terrestrial ecosystem nutrient cycling". Trends in Ecology & Evolution. 15 (6): 238–243. doi:10.1016/S0169-5347(00)01861-9. PMID 10802549.
- Hart, John H.; Hillis, W. E. (1974). "Inhibition of wood-rotting fungi by stilbenes and other polyphenols in Eucalyptus sideroxylon". Phytopathology. 64 (7): 939–948. doi:10.1094/Phyto-64-939.
- Popa, V; Dumitru, M; Volf, I; Anghel, N (2008). "Lignin and polyphenols as allelochemicals". Industrial Crops and Products. 27 (2): 144–149. doi:10.1016/j.indcrop.2007.07.019.
- Nakai, S (2000). "Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa". Water Research. 34 (11): 3026–3032. doi:10.1016/S0043-1354(00)00039-7.
- Wigglesworth, V. B. (1988). "The source of lipids and polyphenols for the insect cuticle: The role of fat body, oenocytes and oenocytoids". Tissue and Cell. 20 (6): 919–932. doi:10.1016/0040-8166(88)90033-X. PMID 18620248.
- Dennell, R. (1947). "The Occurrence and Significance of Phenolic Hardening in the Newly Formed Cuticle of Crustacea decapoda". Proceedings of the Royal Society B: Biological Sciences. 134 (877): 485–503. Bibcode:1947RSPSB.134..485D. doi:10.1098/rspb.1947.0027. PMID 20265564.
- Locke, M.; Krishnan, N. (1971). "The distribution of phenoloxidases and polyphenols during cuticle formation". Tissue and Cell. 3 (1): 103–126. doi:10.1016/S0040-8166(71)80034-4. PMID 18631545.
- Krishnan, G. (September 1951). "Phenolic Tanning and Pigmentation of the Cuticle in Carcinus maenas". Quarterly Journal of Microscopical Science. 92 (19): 333–342.
- Krishnan, G. (September 1954). "The Epicuticle of an Arachnid, Palamneus swammerdami". Quarterly Journal of Microscopical Science. 95 (31): 371–381.
- Tanner, Gregory J; Francki, Kathy T; Abrahams, Sharon; Watson, John M; Larkin, Philip J; Ashton, Anthony R (2003). "Proanthocyanidin Biosynthesis in Plants". Journal of Biological Chemistry. 278 (34): 31647–31656. doi:10.1074/jbc.M302783200. PMID 12788945.
- Krasnow, M. N.; Murphy, T. M. (2004). "Polyphenol Glucosylating Activity in Cell Suspensions of Grape (Vitis vinifera)". Journal of Agricultural and Food Chemistry. 52 (11): 3467–3472. doi:10.1021/jf035234r. PMID 15161217.
- Malek, S. R. A. (1961). "Polyphenols and their quinone derivatives in the cuticle of the desert locust, Schistocerca gregaria (Forskål)". Comparative Biochemistry and Physiology. 2: 35–77. doi:10.1016/0010-406X(61)90071-8.
- Pandey, K. B.; Rizvi, S. I. (2009). "Plant polyphenols as dietary antioxidants in human health and disease". Oxidative Medicine and Cellular Longevity. 2 (5): 270–278. doi:10.4161/oxim.2.5.9498. PMC 2835915. PMID 20716914.
- d'Archivio, M; Filesi, C; Varì, R; Scazzocchio, B; Masella, R (2010). "Bioavailability of the Polyphenols: Status and Controversies". International Journal of Molecular Sciences. 11 (4): 1321–1342. doi:10.3390/ijms11041321. PMC 2871118. PMID 20480022.
- L. Mennen; et al. (January 2005). "Risks and Safety of Polyphenol Consumption". Am J Clin Nutr. 81 (1): 3265–3295. doi:10.1093/ajcn/81.1.326S. PMID 15640498.
- Miglio C, Chiavaro E, Visconti A, Fogliano V, Pellegrini N (2008). "Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables". J Agric Food Chem. 56 (1): 139–147. doi:10.1021/jf072304b. PMID 18069785.
- Scalbert, A; Manach, C; Morand, C; Rémésy, C; Jiménez, L (2005). "Dietary polyphenols and the prevention of diseases". Critical Reviews in Food Science and Nutrition. 45 (4): 287–306. doi:10.1080/1040869059096. ISSN 1040-8398. PMID 16047496. S2CID 15475614.
- "Guidance for Industry: Food Labeling; Nutrient Content Claims; Definition for "High Potency" and Definition for "Antioxidant" for Use in Nutrient Content Claims for Dietary Supplements and Conventional Foods; Small Entity Compliance Guide". Center for Food Safety and Applied Nutrition, US Food and Drug Administration. July 2008. Retrieved 2 October 2017.
- Gross, Paul (1 March 2009). "New Roles for Polyphenols. A 3-Part Report on Current Regulations and the State of Science". Nutraceuticals World.
- Halliwell B (2007). "Dietary polyphenols: Good, bad, or indifferent for your health?". Cardiovasc Res. 73 (2): 341–347. doi:10.1016/j.cardiores.2006.10.004. PMID 17141749.
- "Scientific Opinion on the substantiation of health claims related to: flavonoids and ascorbic acid in fruit juices, including berry juices (ID 1186); flavonoids from citrus (ID 1471); flavonoids from Citrus paradisi Macfad. (ID 3324, 3325); flavonoids (ID". EFSA Journal. 9 (4): 2082. April 2011. doi:10.2903/j.efsa.2011.2082. Lay summary.
- "Scientific Opinion on the modification of the authorisation of a health claim related to cocoa flavanols and maintenance of normal endothelium‐dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006 following a request in accordance with Article 19 of Regulation (EC) No 1924/2006". EFSA Journal. 12 (5). May 2014. doi:10.2903/j.efsa.2014.3654.
- "Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), mainte". EFSA Journal. 9 (4): 2033. April 2011. doi:10.2903/j.efsa.2011.2033.
- Staff, Sensory Society. Basic Tastes: Astringency Archived 27 September 2013 at the Wayback Machine
- Lesschaeve I, Noble AC (2005). "Polyphenols: factors influencing their sensory properties and their effects on food and beverage preferences". Am J Clin Nutr. 81 (1): 330S–335S. doi:10.1093/ajcn/81.1.330S. PMID 15640499.
- Hufnagel JC, Hofmann T (2008). "Orosensory-directed identification of astringent mouthfeel and bitter-tasting compounds in red wine". J Agric Food Chem. 56 (4): 1376–1386. doi:10.1021/jf073031n. PMID 18193832.
- Owen, R. W.; Haubner, R.; Hull, W. E.; Erben, G.; Spiegelhalder, B.; Bartsch, H.; Haber, B. (2003). "Isolation and structure elucidation of the major individual polyphenols in carob fibre". Food and Chemical Toxicology. 41 (12): 1727–1738. doi:10.1016/S0278-6915(03)00200-X. PMID 14563398.
- Escribano-Bailon, Maria Teresa; Santos-Buelga, Celestino (2003). "Polyphenol Extraction From Foods" (PDF). In Santos-Buelga, Celestino; Williamson, Gary (eds.). Methods in Polyphenol Analysis. Royal Society of Chemistry. pp. 1–16. ISBN 978-0-85404-580-8.
- Pan, X (2003). "Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves". Chemical Engineering and Processing. 42 (2): 129–133. doi:10.1016/S0255-2701(02)00037-5.
- Palma, M; Taylor, L (1999). "Extraction of polyphenolic compounds from grape seeds with near critical carbon dioxide". Journal of Chromatography A. 849 (1): 117–124. doi:10.1016/S0021-9673(99)00569-5. PMID 10444839.
- Alonsosalces, R; Korta, E; Barranco, A; Berrueta, L; Gallo, B; Vicente, F (2001). "Pressurized liquid extraction for the determination of polyphenols in apple". Journal of Chromatography A. 933 (1–2): 37–43. doi:10.1016/S0021-9673(01)01212-2. PMID 11758745.
- Sineiro, J.; Domínguez, H.; Núñez, M. J.; Lema, J. M. (1996). "Ethanol extraction of polyphenols in an immersion extractor. Effect of pulsing flow". Journal of the American Oil Chemists' Society. 73 (9): 1121–1125. doi:10.1007/BF02523372. S2CID 96009875.
- Arranz, Sara; Saura-Calixto, Fulgencio; Shaha, Shika; Kroon, Paul A. (2009). "High Contents of Nonextractable Polyphenols in Fruits Suggest That Polyphenol Contents of Plant Foods Have Been Underestimated". Journal of Agricultural and Food Chemistry. 57 (16): 7298–7303. doi:10.1021/jf9016652. hdl:10261/82508. PMID 19637929.
- Nawaz, H; Shi, J; Mittal, G; Kakuda, Y (2006). "Extraction of polyphenols from grape seeds and concentration by ultrafiltration". Separation and Purification Technology. 48 (2): 176–181. doi:10.1016/j.seppur.2005.07.006.
- Ferrara BT, Thompson EP (February 2019). "A method for visualizing fluorescence of flavonoid therapeutics in vivo in the model eukaryote Dictyostelium discoideum". BioTechniques (Paper). 66 (2): 65–71. doi:10.2144/btn-2018-0084. PMID 30744410.

- Tempel, A. S. (1982). "Tannin-measuring techniques". Journal of Chemical Ecology. 8 (10): 1289–1298. doi:10.1007/BF00987762. PMID 24414735. S2CID 39848160.
- Gani, M.; McGuinness, B. J.; Da Vies, A. P. (1998). "Monoclonal antibodies against tea polyphenols: A novel immunoassay to detect polyphenols in biological fluids". Food and Agricultural Immunology. 10: 13–22. doi:10.1080/09540109809354964.
- Walker, Richard B.; Everette, Jace D. (2009). "Comparative Reaction Rates of Various Antioxidants with ABTS Radical Cation". Journal of Agricultural and Food Chemistry. 57 (4): 1156–1161. doi:10.1021/jf8026765. PMID 19199590.
- Roy, Molay K; Koide, Motoki; Rao, Theertham P; Okubo, Tsutomu; Ogasawara, Yutaka; Juneja, Lekh R (2010). "ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content". International Journal of Food Sciences and Nutrition. 61 (2): 109–124. doi:10.3109/09637480903292601. PMID 20109129. S2CID 1929167.
- Pulido, R.; Bravo, L.; Saura-Calixto, F. (2000). "Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay". Journal of Agricultural and Food Chemistry. 48 (8): 3396–3402. doi:10.1021/jf9913458. hdl:10261/112476. PMID 10956123.
- Meyer, A. S.; Yi, O. S.; Pearson, D. A.; Waterhouse, A. L.; Frankel, E. N. (1997). "Inhibition of Human Low-Density Lipoprotein Oxidation in Relation to Composition of Phenolic Antioxidants in Grapes (Vitis vinifera)". Journal of Agricultural and Food Chemistry. 45 (5): 1638–1643. doi:10.1021/jf960721a.
- Mello, L; Sotomayor, Maria Del Pilar Taboada; Kubota, Lauro Tatsuo (2003). "HRP-based amperometric biosensor for the polyphenols determination in vegetables extract". Sensors and Actuators B: Chemical. 96 (3): 636–645. doi:10.1016/j.snb.2003.07.008.
- "Polyphenol". Merriam-Webster, Inc. 2019. Retrieved 23 February 2019.
External links
| Look up polyphenol in Wiktionary, the free dictionary. |