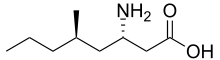
Imagabalin
Imagabalin (INN, USAN; PD-0332334) was an investigational drug that acted as a ligand for the α2δ subunit of the voltage-dependent calcium channel,[1] with some selectivity for the α2δ1 subunit over α2δ2.[2] It was under development by Pfizer as a pharmaceutical medication due to its hypothesized anxiolytic, analgesic, hypnotic, and anticonvulsant-like activity. It reached phase-III clinical trials for treatment of generalized anxiety disorder; however, the trials were terminated by the manufacturer.[3][4][5][6] The drug is no longer under development.
 | |
| Clinical data | |
|---|---|
| Other names | PD-0332334; PD-332,334 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H19NO2 |
| Molar mass | 173.256 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Quintero JE, Pomerleau F, Huettl P, Johnson KW, Offord J, Gerhardt GA (July 2011). "Methodology for rapid measures of glutamate release in rat brain slices using ceramic-based microelectrode arrays: basic characterization and drug pharmacology". Brain Research. 1401: 1–9. doi:10.1016/j.brainres.2011.05.025. PMC 3197737. PMID 21664606.
- Larry Ereshefsky (2008). "Therapies in the Pipeline for Sleep Disorders: Focus on Novel Mechanisms and Disease Models" (PDF) (Press release). p. 11. Retrieved 2012-04-22.
- Pfizer (2012-11-09). "A Phase 3, Randomized, Double-Blind, Parallel Group, 10-Week Placebo Controlled Fixed Dose Study Of PD 0332334 And Paroxetine Evaluating The Efficacy And Safety Of PD 0332334 For The Treatment Of Generalized Anxiety Disorder". Cite journal requires
|journal=(help) - Pfizer (2012-11-09). "A Phase 3, Randomized, Double-Blind, Parallel Group, 10-Week Placebo Controlled Fixed Dose Study Of PD 0332334 And Paroxetine Evaluating The Efficacy And Safety Of PD 0332334 For The Treatment Of Generalized Anxiety Disorder". Cite journal requires
|journal=(help) - Pfizer (2012-11-09). "A Phase 3, Randomized, Double-Blind, Parallel Group, 10-Week Placebo Controlled Fixed Dose Study of PD 0332334 and Paroxetine Evaluating the Efficacy and Safety of PD 0332334 for the Treatment of Generalized Anxiety Disorder". Cite journal requires
|journal=(help) - Pfizer (2012-11-09). "A 52-Week Open-Label Safety Study of PD 0332334 in Subjects With Generalized Anxiety Disorder". Cite journal requires
|journal=(help)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.