WHO Model List of Essential Medicines for Children
The WHO Model List of Essential Medicines for Children (aka Essential Medicines List for Children[1] or EMLc[1]) is a list, proposed by the World Health Organization (WHO), of the most effective and safe medicines for use in children up to 12 years of age needed to meet the most important needs in a basic health-care system.[2] The list is divided into core items and complementary items. The core items are deemed to be the most cost effective options for key health problems and are usable with little additional health care resources. The complementary items frequently require additional infrastructure such as specially trained health care providers or diagnostic equipment. The first list for children was created in 2007, and the list is in its 7th edition as of 2019.[3][4]
Note: In the following article, an α indicates the medicine is a complementary item, for which specialized diagnostic or monitoring and/or specialist training are needed. An item may also be listed as complementary on the basis of higher costs and/or a less attractive cost/benefit ratio.
Anaesthetics
Inhalational medicines
Local anaesthetics
Medicines for pain and palliative care
Non-opioids and non-steroidal anti-inflammatory medicines (NSAIMs)
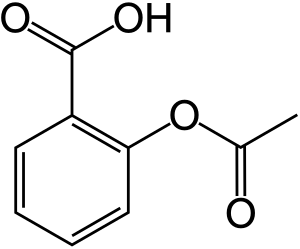
- Ibuprofen
- Paracetamol[note 3] (acetaminophen)
Medicines for other symptoms common in palliative care
Antiallergics and medicines used in anaphylaxis
- Dexamethasone
- Epinephrine (adrenaline)
- Hydrocortisone
- Loratadine[note 5]
- Prednisolone
Antidotes and other substances used in poisonings
Non-specific
Anticonvulsants/antiepileptics
- Carbamazepine
- Diazepam
- Lamotrigine[note 6]
- Lorazepam
- Midazolam[note 7]
- Phenobarbital
- Phenytoin
- Valproic acid (sodium valproate)
- Ethosuximideα
- Valproic acid (sodium valproate)α
Anti-infective medicines
Intestinal antihelminthics
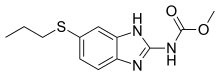
Antifilarials
Antischistosomals and other antinematode medicines
Access group antibiotics
- Amikacin
- Amoxicillin
- Amoxicillin/clavulanic acid (amoxicillin + clavulanic acid)
- Ampicillin
- Benzathine benzylpenicillin
- Benzylpenicillin
- Cefalexin
- Cefazolin
- Chloramphenicol
- Clindamycin
- Cloxacillin[note 8]
- Doxycycline
- Gentamicin
- Metronidazole
- Nitrofurantoin
- Phenoxymethylpenicillin (penicillin V)
- Procaine benzylpenicillin[note 9]
- Sulfamethoxazole/trimethoprim[note 10] (sulfamethoxazole + trimethoprim)
Watch group antibiotics
Reserve group antibiotics
Antileprosy medicines
Antituberculosis medicines

- Ethambutol
- Isoniazid
- Isoniazid/pyrazinamide/rifampicin (isoniazid + pyrazinamide + rifampicin)
- Isoniazid/rifampicin (isoniazid + rifampicin)
- Pyrazinamide
- Rifampicin
- Rifapentine[note 15]
- Amikacinα
- Amoxicillin/clavulanic acidα (amoxicillin + clavulanic acid)
- Bedaquilineα
- Clofazimineα
- Cycloserineα
- Delamanidα
- Ethionamideα[note 16]
- Levofloxacinα
- Linezolidα
- Meropenemα
- Moxifloxacinα
- P-aminosalicylic acidα
- Streptomycinα
Antifungal medicines
Antiherpes medicines
Nucleoside/nucleotide reverse transcriptase inhibitors
- Abacavir (ABC)
- Lamivudine (3TC)
- Zidovudine (ZDV or AZT)
Non-nucleoside reverse transcriptase inhibitors
- Efavirenz (EGV or EFZ)
- Nevirapine (NVP)
Protease inhibitors
200mg.jpg.webp)
- Atazanavir
- Darunavir
- Lopinavir/ritonavir (LPV/r) (lopinavir + ritonavir)
- Ritonavir
Integrase inhibitors
Medicines for prevention of HIV-related opportunistic infections
Other antivirals
Nucleoside/Nucleotide reverse transcriptase inhibitors
Medicines for hepatitis C
No listings in this section.
Antiamoebic and antigiardiasis medicines
Antileishmaniasis medicines
For curative treatment
- Amodiaquine[note 23]
- Artemether[note 24]
- Artemether/lumefantrine[note 25]
- Artesunate[note 26]
- Artesunate/amodiaquine[note 27]
- Artesunate/mefloquine
- Artesunate/pyronaridine tetraphosphate
- Chloroquine[note 28]
- Dihydroartemisinin/piperaquine phosphate
- Doxycycline[note 29]
- Mefloquine[note 30]
- Primaquine[note 31]
- Quinine[note 32]
- Sulfadoxine/pyrimethamine[note 33]
For chemoprevention
Antipneumocystosis and antitoxoplasmosis medicines
African trypanosomiasis
1st stage
American trypanosomiasis
Medicines for ectoparasitic infections
Antimigraine medicines
For treatment of acute attack
For prophylaxis
Immunomodulators and Antineoplastics
Immunomodulators for non-malignant disease
Cytotoxic medicines
- Arsenic trioxideα
- Asparaginaseα
- Bleomycinα
- Calcium folinateα
- Carboplatinα
- Cisplatinα
- Cyclophosphamideα
- Cytarabineα
- Dacarbazineα
- Dactinomycinα
- Daunorubicinα
- Doxorubicinα
- Etoposideα
- Fluorouracilα
- Hydroxycarbamideα
- Ifosfamideα
- Irinotecanα
- Mercaptopurineα
- Methotrexateα
- Oxaliplatinα
- Paclitaxelα
- Pegaspargaseα[note 41]
- Procarbazineα
- Realgar/Indigo naturalisα
- Tioguanineα
- Vinblastineα
- Vincristineα
Targeted therapies
Immunomodulators
Hormones and antihormones
Supportive medicines
Antiparkinsonism
No listings in this section.
Medicines affecting the blood
Antianaemia medicines
Medicines affecting coagulation
Other medicines for haemoglobinopathies
Blood products of human origin and plasma substitutes
Blood and blood components

Human immunoglobulins
Blood coagulation factors
Plasma substitutes
Cardiovascular medicines
Antianginal medicines
No listings in this section.
Antiarrhythmic medicines
No listings in this section.
Antihypertensive medicines
Medicines used in heart failure
Antithrombotic medicines
No listings in this section.
Lipid-lowering agents
No listings in this section.
Dermatological medicines (topical)
Antifungal medicines
Anti-infective medicines
Anti-inflammatory and antipruritic medicines
Medicines affecting skin differentiation and proliferation
- Benzoyl peroxide
- Coal tar
- Podophyllum resin
- Salicylic acid
- Urea
Scabicides and pediculicides
Gastrointestinal medicines
Antiulcer medicines
Antiemetic medicines
Anti-inflammatory medicines
No listings in this section.
Laxatives
No listings in this section.
Medicines used in diarrhoea
- Oral rehydration salts + zinc sulfate (Co-packaged)
Oral rehydration
Medicines for diarrhoea
Medicines for endocrine disorders
Adrenal hormones and synthetic substitutes
Androgens
No listings in this section.
Estrogens
No listings in this section.
Progestogens
No listings in this section.
Insulins
- Insulin injection (soluble)
- Intermediate-acting insulin
Thyroid hormones and antithyroid medicines
Immunologicals
Diagnostic agents
- Tuberculin, purified protein derivative (PPD)
Sera and immunoglobulins
Vaccines
- BCG vaccine
- Diphtheria vaccine
- Haemophilus influenzae type b vaccine
- Hepatitis B vaccine
- HPV vaccine
- Measles vaccine
- Pertussis vaccine
- Pneumococcal vaccine
- Poliomyelitis vaccine
- Rotavirus vaccine
- Rubella vaccine
- Tetanus vaccine
- Japanese encephalitis vaccine[note 51]
- Yellow fever vaccine[note 51]
- Tick-borne encephalitis vaccine[note 51]
- Cholera vaccine[note 52]
- Dengue vaccine[note 52]
- Hepatitis A vaccine[note 52]
- Meningococcal meningitis vaccine[note 52]
- Rabies vaccine[note 52]
- Typhoid vaccine[note 52]
- Influenza vaccine[note 53]
- Mumps vaccine[note 53]
- Varicella vaccine[note 53]
Muscle relaxants (peripherally-acting) and cholinesterase inhibitors
Ophthalmological preparations
Anti-infective agents
Anti-inflammatory agents
Local anaesthetics
Miotics and antiglaucoma medicines
No listings in this section.
Mydriatics
- Atropine[note 55]
- Epinephrine (adrenaline)α
Anti-vascular endothelial growth factor (VEGF) preparations
No listings in this section.
Medicines for reproductive health and perinatal care
Contraceptives
No listings in this section.
Ovulation inducers
No listings in this section.
Uterotonics
No listings in this section.
Antioxytocics (tocolytics)
No listings in this section.
Other medicines administered to the mother
No listings in this section.
Medicines administered to the neonate
- Caffeine citrate
- Chlorhexidine
- Ibuprofenα
- Prostaglandin Eα
- Surfactantα
Peritoneal dialysis solution
- Intraperitoneal dialysis solution (of appropriate composition)α
Medicines for mental and behavioural disorders
Medicines used in psychotic disorders
Medicines used in depressive disorders
Medicines used in bipolar disorders
No listings in this section.
Medicines for anxiety disorders
No listings in this section.
Medicines used for obsessive compulsive disorders
No listings in this section.
Medicines for disorders due to psychoactive substance use
No listings in this section.
Medicines acting on the respiratory tract
Antiasthmatic medicines
- Budesonide
- Epinephrine (adrenaline)
- Salbutamol (albuterol)
Solutions correcting water, electrolyte and acid-base disturbances
Parenteral
Miscellaneous
Vitamins and minerals
Ear, nose and throat medicines
Medicines for diseases of joints
Medicines used to treat gout
No listings in this section.
Disease-modifying agents used in rheumatoid disorders
Notes
- Thiopental may be used as an alternative depending on local availability and cost.
- No more than 30% oxygen should be used to initiate resuscitation of neonates less than or equal to 32 weeks of gestation.
- Not recommended for anti‐inflammatory use due to lack of proven benefit to that effect.
- Alternatives limited to hydromorphone and oxycodone.
- There may be a role for sedating antihistamines for limited indications.
- as adjunctive therapy for treatment-resistant partial or generalized seizures.
- for buccal administration when solution for oromucosal administration is not available
- cloxacillin, dicloxacillin and flucloxacillin are preferred for oral administration due to better bioavailability.
- Procaine benzylpenicillin is not recommended as first-line treatment for neonatal sepsis except in settings with high neonatal mortality, when given by trained health workers in cases where hospital care is not achievable.
- single agent trimethoprim may be an alternative for lower urinary tract infection.
- also listed for single-dose treatment of trachoma and yaws.
- 3rd generation cephalosporin of choice for use in hospitalised neonates.
- Do not administer with calcium and avoid in infants with hyperbilirubinaemia.
- erythromycin may be an alternative
- For treatment of latent TB infection (LTBI) only
- Prothionamide may be used as an alternative.
- For treatment of chronic pulmonary aspergillosis, acute invasive aspergillosis, histoplasmosis, sporotrichosis, paracoccidiodomycosis, mycoses caused by T. marneffei and chromoblastomycosis; and prophylaxis of histoplasmosis and infections caused by T. marneffei in AIDS patients.
- For treatment of chronic pulmonary aspergillosis and acute invasive aspergillosis.
- for use in second-line regimens in accordance with WHO treatment guidelines
- For the treatment of viral haemorrhagic fevers only.
- Severe illness due to confirmed or suspected influenza virus infection in critically ill hospitalized patients
- For the treatment of cytomegalovirus retinitis (CMVr).
- To be used in combination with artesunate 50 mg.
- For use in the management of severe malaria.
- Not recommended in the first trimester of pregnancy or in children below 5 kg.
- To be used in combination with either amodiaquine, mefloquine or sulfadoxine + pyrimethamine.
- Other combinations that deliver the target doses required such as 153 mg or 200 mg (as hydrochloride) with 50 mg artesunate can be alternatives.
- For use only for the treatment of P.vivax infection.
- For use only in combination with quinine.
- To be used in combination with artesunate 50 mg.
- Only for use to achieve radical cure of P.vivax and P.ovale infections, given for 14 days.
- For use only in the management of severe malaria, and should be used in combination with doxycycline.
- Only in combination with artesunate 50 mg.
- For use only for the treatment of P.vivax infection.
- For use only in combination with chloroquine.
- For the treatment of 1st and 2nd stage of human African trypanosomiasis due to Trypanosoma brucei gambiense infection.
- To be used for the treatment of Trypanosoma brucei gambiense infection.
- To be used for the treatment of the initial phase of Trypanosoma brucei rhodesiense infection.
- To be used for the treatment of Trypanosoma brucei gambiense infection
- Only to be used in combination with eflornithine, for the treatment of Trypanosoma brucei gambiense infection.
- including quality-assured biosimilars
- including quality-assured biosimilars
- the square box applies to epoetin alfa, beta and theta, darbepoetin alfa, and their respective biosimilars
- Alternatives are limited to nadroparin and dalteparin
- Deferasirox oral form may be an alternative, depending on cost and availability.
- Polygeline, injectable solution, 3.5% is considered as equivalent.
- In acute diarrhoea zinc sulfate should be used as an adjunct to oral rehydration salts
- carbimazole is an alternative depending on local availability.
- for use in patients for whom alternative first-line treatment is not appropriate or available
- Exact type to be defined locally.
- Recommended for certain regions
- Recommended for some high-risk populations
- Recommended for immunisation programmes with certain characteristics
- Infections due to Chlamydia trachomatis or Neisseria gonorrhoeae.
- Or homatropine (hydrobromide) or cyclopentolate (hydrochloride).
- Ergocalciferol can be used as an alternative.
- For use for rheumatic fever, juvenile arthritis, Kawasaki disease
References
- "WHO Model Lists of Essential Medicines". WHO.int.
The current versions are the 21st WHO Essential Medicines List (EML) and the 7th WHO Essential Medicines List for Children (EMLc) updated in June 2019.
- "Essential medicines". World Health Organization. Retrieved 20 January 2017.
- World Health Organization (2019). World Health Organization model list of essential medicines for children: 7th list 2019. Geneva. hdl:10665/325772. WHO/MVP/EMP/IAU/2019.07. License: CC BY-NC-SA 3.0 IGO.
- World Health Organization (2019). Executive summary: the selection and use of essential medicines 2019: report of the 22nd WHO Expert Committee on the selection and use of essential medicines. Geneva. hdl:10665/325773. WHO/MVP/EMP/IAU/2019.05. License: CC BY-NC-SA 3.0 IGO.
Further reading
- World Health Organization (2019). The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization. hdl:10665/330668. ISBN 9789241210300. ISSN 0512-3054. WHO technical report series;1021.