Opioid peptide
Opioid peptides are peptides that bind to opioid receptors in the brain; opiates and opioids mimic the effect of these peptides. Such peptides may be produced by the body itself, for example endorphins. The effects of these peptides vary, but they all resemble those of opiates. Brain opioid peptide systems are known to play an important role in motivation, emotion, attachment behaviour, the response to stress and pain, and the control of food intake.
| Vertebrate endogenous opioids neuropeptide | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Opiods_neuropep | ||||||||
| Pfam | PF01160 | ||||||||
| InterPro | IPR006024 | ||||||||
| PROSITE | PDOC00964 | ||||||||
| |||||||||
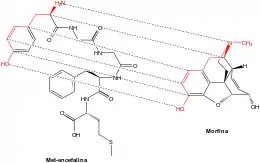
Opioid-like peptides may also be absorbed from partially digested food (casomorphins, exorphins, and rubiscolins). The opioid food peptides have lengths of typically 4–8 amino acids. The body's own opioids are generally much longer.
Opioid peptides are released by post-translational proteolytic cleavage of precursor proteins. The precursors consist of the following components: a signal sequence that precedes a conserved region of about 50 residues; a variable-length region; and the sequence of the neuropeptides themselves. Sequence analysis reveals that the conserved N-terminal region of the precursors contains 6 cysteines, which are probably involved in disulfide bond formation. It is speculated that this region might be important for neuropeptide processing.[1]
Endogenous opioids produced in the body
The human genome contains several homologous genes that are known to code for endogenous opioid peptides.
- The nucleotide sequence of the human gene for proopiomelanocortin (POMC) was characterized in 1980.[2] The POMC gene codes for endogenous opioids such as β-endorphin and γ-endorphin.[3]
- The human gene for the enkephalins was isolated and its sequence described in 1982.[4]
- The human gene for dynorphins (originally called the "Enkephalin B" gene because of sequence similarity to the enkephalin gene) was isolated and its sequence described in 1983.[5]
- The PNOC gene encoding prepronociceptin, which is cleaved into nociceptin and potentially two additional neuropeptides.[1]
- Adrenorphin, amidorphin, and leumorphin were discovered in the 1980s.
- The endomorphins were discovered in the 1990s.
- Opiorphin and spinorphin, enkephalinase inhibitors (i.e., prevent the metabolism of enkephalins).
- Hemorphins, hemoglobin-derived opioid peptides, including hemorphin-4, valorphin, and spinorphin, among others.
While not peptides, codeine and morphine are also produced in the human body.[6][7]
| Opioid peptide | Amino acid sequence | Opioid receptor target(s) | References |
|---|---|---|---|
| Enkephalins | |||
| Leu-enkephalin | YGGFL | δ-opioid receptor†, μ-opioid receptor† | [8][9][10] |
| Met-enkephalin | YGGFM | δ-opioid receptor†, μ-opioid receptor† | [8][9][10] |
| Metorphamide | YGGFMRRV-NH2 | δ-opioid receptor, μ-opioid receptor | [8] |
| Peptide E | YGGFMRRVGRPEWWMDYQKRYGGFL | μ-opioid receptor, κ-opioid receptor | [8] |
| Endorphins | |||
| α-Endorphin | YGGFMTSEKSQTPLVT | μ-opioid receptor, unknown affinity for other opioid receptors | [8] |
| β-Endorphin | YGGFMTSEKSQTPLVTLFKNAIIKNAYKKGE | μ-opioid receptor'†'‡, δ-opioid receptor† | [8][9][10][7] |
| γ-Endorphin | YGGFMTSEKSQTPLVTL | μ-opioid receptor, unknown affinity for other opioid receptors | [8] |
| Dynorphins | |||
| Dynorphin A | YGGFLRRIRPKLKWDNQ | κ-opioid receptor'†'‡ | [8][9][11] |
| Dynorphin A1–8 | YGGFLRRI | κ-opioid receptor, μ-opioid receptor (partial agonist at δ-opioid receptor) | [12][13] |
| Dynorphin B | YGGFLRRQFKVVT | κ-opioid receptor | [8][9] |
| Big dynorphin | YGGFLRRIRPKLKWDNQKRYGGFLRRQFKVVT | κ-opioid receptor'†'‡ | [11][14][15] |
| Leumorphin | YGGFLRRQFKVVTRSQEDPNAYYEELFDV | κ-opioid receptor | [16][17][18][19] |
| α-Neoendorphin | YGGFLRKYPK | κ-opioid receptor | [8][9] |
| β-Neoendorphin | YGGFLRKYP | κ-opioid receptor | [8] |
| Nociceptin | |||
| Nociceptin | FGGFTGARKSARKLANQ | nociceptin receptor'†'‡ | [8][9][20] |
| Endomorphins | |||
| Endomorphin-1 | YPWF-NH2 | μ-opioid receptor | [8][9] |
| Endomorphin-2 | YPFF-NH2 | μ-opioid receptor | [8][9] |
| † This symbol next to a receptor indicates that the corresponding peptide is a principal endogenous agonist of the receptor in humans. ‡ This symbol next to a receptor indicates that the corresponding peptide is the endogenous ligand with the highest known potency for the receptor in humans. | |||
Opioid food peptides
Exogenous opioid substances are called exorphins, as opposed to endorphines. Exorphins include opioid food peptides like Gluten exorphin and opioid food peptides and are mostly contained in cereals and animal milk. They mimic the actions of endorphines because they bind to the same opioid receptors in the brain.
These are the most common exorphins:
- Casomorphin (from casein found in milk of mammals, including cows)
- Gluten exorphin (from gluten found in cereals wheat, rye, barley)
- Gliadorphin/gluteomorphin (from gluten found in cereals wheat, rye, barley)
- Soymorphin-5 (from soybean)
- Rubiscolin (from spinach)
Amphibian opioid peptides
- Deltorphin
- Deltorphin I
- Deltorphin II
- Dermorphin
Synthetic opioid peptides
- Zyklophin – semisynthetic KOR antagonist derived from dynorphin A
References
- Mollereau C, Simons MJ, Soularue P, Liners F, Vassart G, Meunier JC, Parmentier M (August 1996). "Structure, tissue distribution, and chromosomal localization of the prepronociceptin gene". Proc. Natl. Acad. Sci. U.S.A. 93 (16): 8666–70. Bibcode:1996PNAS...93.8666M. doi:10.1073/pnas.93.16.8666. PMC 38730. PMID 8710928.
- Chang AC, Cochet M, Cohen SN (August 1980). "Structural organization of human genomic DNA encoding the pro-opiomelanocortin peptide". Proc. Natl. Acad. Sci. U.S.A. 77 (8): 4890–4. Bibcode:1980PNAS...77.4890C. doi:10.1073/pnas.77.8.4890. PMC 349954. PMID 6254047.
- Ling N, Burgus R, Guillemin R (November 1976). "Isolation, primary structure, and synthesis of alpha-endorphin and gamma-endorphin, two peptides of hypothalamic-hypophysial origin with morphinomimetic activity". Proc. Natl. Acad. Sci. U.S.A. 73 (11): 3942–6. Bibcode:1976PNAS...73.3942L. doi:10.1073/pnas.73.11.3942. PMC 431275. PMID 1069261.
- Noda M, Teranishi Y, Takahashi H, Toyosato M, Notake M, Nakanishi S, Numa S (June 1982). "Isolation and structural organization of the human preproenkephalin gene". Nature. 297 (5865): 431–4. Bibcode:1982Natur.297..431N. doi:10.1038/297431a0. PMID 6281660. S2CID 4371340.
- Horikawa S, Takai T, Toyosato M, Takahashi H, Noda M, Kakidani H, et al. (December 1983). "Isolation and structural organization of the human preproenkephalin B gene". Nature. 306 (5943): 611–4. Bibcode:1983Natur.306..611H. doi:10.1038/306611a0. PMID 6316163. S2CID 4315441.
- Stefano GB, Ptáček R, Kuželová H, Kream RM (2012). "Endogenous morphine: up-to-date review 2011" (PDF). Folia Biol. (Praha). 58 (2): 49–56. PMID 22578954.
Positive evolutionary pressure has apparently preserved the ability to synthesize chemically authentic morphine, albeit in homeopathic concentrations, throughout animal phyla. ... The apparently serendipitous finding of an opiate alkaloid-sensitive, opioid peptide-insensitive, µ3 opiate receptor subtype expressed by invertebrate immunocytes, human blood monocytes, macrophage cell lines, and human blood granulocytes provided compelling validating evidence for an autonomous role of endogenous morphine as a biologically important cellular signalling molecule (Stefano et al., 1993; Cruciani et al., 1994; Stefano and Scharrer, 1994; Makman et al., 1995). ... Human white blood cells have the ability to make and release morphine
- "μ receptor". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. 15 March 2017. Retrieved 28 December 2017.
Comments: β-Endorphin is the highest potency endogenous ligand ... Morphine occurs endogenously [117].. ...
Principal endogenous agonists (Human)
β-endorphin (POMC, P01189), [Met]enkephalin (PENK, P01210), [Leu]enkephalin (PENK, P01210) - Li Y, Lefever MR, Muthu D, Bidlack JM, Bilsky EJ, Polt R (February 2012). "Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins". Future Medicinal Chemistry. 4 (2): 205–226. doi:10.4155/fmc.11.195. PMC 3306179. PMID 22300099.
Table 1: Endogenous opioid peptides
- Toll L, Caló G, Cox BM, Chavkin C, Christie MJ, Civelli O, Connor M, Devi LA, Evans C, Henderson G, Höllt V, Kieffer B, Kitchen I, Kreek MJ, Liu-Chen LY, Meunier JC, Portoghese PS, Shippenberg TS, Simon EJ, Traynor JR, Ueda H, Wong YH (10 August 2015). "Opioid receptors: Introduction". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. Retrieved 20 October 2017.
- "δ receptor". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. 15 May 2017. Retrieved 28 December 2017.
Principal endogenous agonists (Human)
β-endorphin (POMC, P01189), [Leu]enkephalin (PENK, P01210), [Met]enkephalin (PENK, P01210) - "κ receptor". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. 21 February 2017. Retrieved 28 December 2017.
Comments: Dynorphin A and big dynorphin are the highest potency endogenous ligands ...
Principal endogenous agonists (Human)
big dynorphin (PDYN, P01213), dynorphin A (PDYN, P01213) - "Dynorphin A 1–8". HMDB Version 4.0. Human Metabolome Database. 27 September 2017. Retrieved 20 October 2017.
Dynorphin A (1–8) is a fraction of Dynorphin A with only Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile peptide chain.
- "Dynorphin A-(1–8): Biological activity". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. Retrieved 20 October 2017.
- "Big dynorphin: Biological activity". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. Retrieved 20 October 2017.
Principal endogenous agonists at κ receptor
- "Big dynorphin: Structure – Peptide Sequence". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. Retrieved 20 October 2017.
Peptide sequence
YGGFLRRIRPKLKWDNQKRYGGFLRRQFKVVT - Schwarzer C (September 2009). "30 years of dynorphins—new insights on their functions in neuropsychiatric diseases". Pharmacology & Therapeutics. 123 (3): 353–370. doi:10.1016/j.pharmthera.2009.05.006. PMC 2872771. PMID 19481570.
- "Dynorphin B (1-29)". PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. 23 December 2017. Retrieved 28 December 2017.
- Suda M, Nakao K, Yoshimasa T, Sakamoto M, Morii N, Ikeda Y, Yanaihara C, Yanaihara N, Numa S, Imura H (September 1984). "Human leumorphin is a potent, kappa opioid receptor agonist". Neuroscience Letters. 50 (1–3): 49–52. doi:10.1016/0304-3940(84)90460-9. PMID 6149506. S2CID 42419724.
- Inenaga K, Nagatomo T, Nakao K, Yanaihara N, Yamashita H (January 1994). "Kappa-selective agonists decrease postsynaptic potentials and calcium components of action potentials in the supraoptic nucleus of rat hypothalamus in vitro". Neuroscience. 58 (2): 331–340. doi:10.1016/0306-4522(94)90039-6. PMID 7908725. S2CID 24631286.
- "NOP receptor". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. 18 August 2017. Retrieved 28 December 2017.
Natural/Endogenous Ligands
nociceptin/orphanin FQ
External links
- Opioid+Peptides at the US National Library of Medicine Medical Subject Headings (MeSH)