21-Hydroxylase
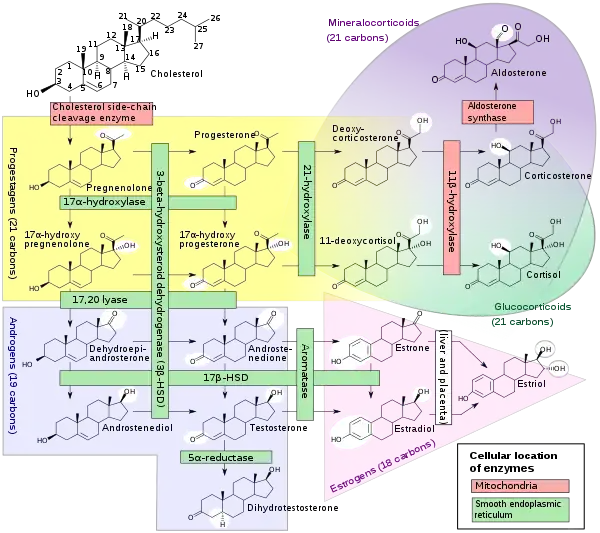
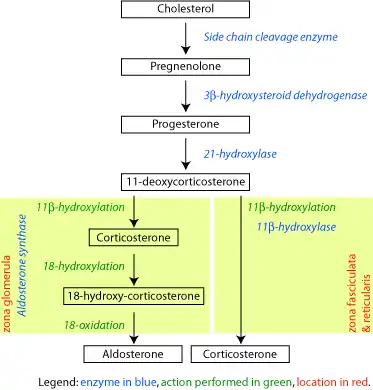
Steroid 21-hydroxylase, also called steroid 21-monooxygenase,[1] 21α-hydroxylase,[2] and, less commonly, 21β-hydroxylase,[3][4] is an enzyme that hydroxylates steroids at the C21 position[5][6] and is involved in biosynthesis of aldosterone and cortisol. The enzyme converts progesterone and 17α-hydroxyprogesterone into 11-deoxycorticosterone and 11-deoxycortisol, respectively.[7][8] The products of the conversions then continue through their appropriate pathways towards creation of aldosterone and cortisol. The enzyme is localized in endoplasmic reticulum membranes of adrenal cortex,[9][10] and is encoded by CYP21A2 gene in humans.
| Steroid 21-hydroxylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.14.14.16 | ||||||||
| CAS number | 9029-68-9 | ||||||||
| Alt. names | P45021A2, CYP21A2, steroid 21-hydroxylase, 21-hydroxylase, 21α-hydroxylase, 21β-hydroxylase | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Function
The steroid 21-hydroxylase (or simply 21-hydroxylase) enzyme is called this way because it hydroxylates steroids at the C21 position.[8] The enzyme catalyzes the chemical reaction in which the hydroxyl group (-OH) is added at the C21 position of the steroid biomolecule. The enzyme is a member of the cytochrome P450 superfamily of monooxygenase enzymes. The cytochrome P450 enzymes catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. 21-hydroxylase is localized in microsomes of endoplasmic reticulum membranes within adrenal cortex. It is one of three microsomal steroidogenic P450 enzymes, the others being 17-hydroxylase and aromatase.[11]
21-hydroxylase is an essential enzyme in the biosynthetic pathways that produce cortisol and aldosterone.[12][13]
Structure
21-hydroxylase is a complex of three independent and identical enzyme subunits. Each subunit in the human enzyme consists of 13 α-helices and 9 ß-strands, formed into a triangular prism-like tertiary structure.[7] The iron(III) heme group that defines the active site resides in the center of each subunit. The human enzyme binds one substrate at a time.[7] In contrast, the well-characterized bovine enzyme can bind two substrates.[14] The human and bovine enzyme share 80% amino acid sequence identity, but are structurally different, particularly in loop regions, and also evident in secondary structure elements.[7]
Reaction
21-Hydroxylase catalyzes the addition of hydroxyl (-OH) to the C21 position of two steroids: progesterone and 17α-hydroxyprogesterone. This reaction was first described in 1952.[15]


Mechanism
21-Hydroxylase is a cytochrome P450 enzyme and follows the P450 catalytic cycle.
21-Hydroxylase is highly specific for hydroxylation of progesterone and 17-hydroxyprogesterone. This is in marked contrast to the evolutionarily and functionally related P450 enzyme 17-hydroxylase, which has a broad range of substrates.[16]
Earlier studies of the human enzyme expressed in yeast classified 17-hydroxyprogesterone as the preferred substrate for 21-hydroxylase.[16][17][18] However, more recent analysis of the purified human enzyme found a lower KM and greater catalytic efficiency for progesterone over 17-hydroxyprogesterone.[7]
The catalytic efficiency of 21-hydroxylase for conversion of progesterone in humans is approximately 1.3 x 10^7 M-1s-1 at 37 °C. This makes it the most catalytically efficient P450 enzyme of those reported to date, and catalytically more efficient than the closely related bovine 21-hydroxylase enzyme.[9] C-H bond breaking to create a primary carbon radical is thought to be the rate-limiting step in the hydroxylation.[7]
Like other cytochrome P450 enzymes, 21-hydroxylase participates in the cytochrome P450 catalytic cycle and engages in one-electron transfer with NADPH-P450 reductase. Its structure includes an essential iron heme group centered within the protein, also common to all P450 enzymes. Variations of the 21-hydroxylase enzyme can be found in all vertebrates.[19] However, understanding of human 21-hydroxylase structure and function is of particular clinical value, as a failure of the enzyme to act appropriately results in congenital adrenal hyperplasia. The X-ray crystal structure for human 21-hydroxylase, with bound progesterone, was realized and published in 2015, providing opportunity for further study.[7] The enzyme is notable for its substrate specificity and relatively high catalytic efficiency.
Genetics
Location
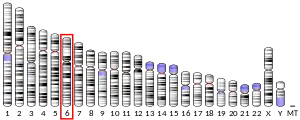
21-hydroxylase is a protein encoded by CYP21A2 gene in humans. A related pseudogene, CYP21A1P, is located near this gene.[24] Both genes are located on chromosome 6, in the major histocompatibility complex III[25] close to the Complement component 4 genes C4A and C4B, the Tenascin X gene TNXB and STK19,[26] and the pseudogene, CYP21A1P, retains 98% exonic sequence identity with the functional gene CYP21A2.[27]
In the mouse genome, the CYP21A2 is a pseudogene and the CYP21A1 is a functional gene.[28] In the chicken and quail, there is only a single CYP21 gene, which locus is located between complement component C4 and TNX gene, along with CENPA.[29]

Role in the human major histocompatibility complex
MHC class III is a group of proteins belonging to the class of major histocompatibility complex (MHC).[25] Unlike other MHC types such as MHC class I and MHC class II, of which their structure and functions in immune response are well defined, MHC class III are poorly defined structurally and functionally. It covers 700 kb and contains 61 genes, making it the most gene-dense region of the human genome.[30] The functions of many genes are yet unknown.
The CYP21A2 gene travels in tandem with a pseudogene, CYP21P1, and the high degree of sequence similarity between them indicates that these two genes are evolving in tandem through intergenic exchange of DNA.[31] The CYP21A2 gene is located within the RCCX cluster, which is the most complex gene cluster in the human genome.[32] Due to the high degree of homology between the CYP21A2 gene and the CYP21P1 pseudogene and the complexity of the locus, it is difficult to study the CYP21A2 gene at the molecular level.[33]
Clinical significance
Congenital adrenal hyperplasia
Genetic variants in the CYP21A2 gene cause a disturbance of the development of the enzyme, which may lead to congenital adrenal hyperplasia due to 21-hydroxylase deficiency. A related pseudogene CYP21A1 is located near this gene. Gene conversion events involving the functional gene and the pseudogene are thought to account for many cases of steroid 21-hydroxylase deficiency.[5] Congenital adrenal hyperplasia (CAH) is an autosomal recessive disorder. There are multiple forms of CAH, broken down into classical and nonclassical forms based on the amount of function retained. The classical forms occurs in approximately 1 in 15000 births globally,[34][35] and include salt-wasting (SW), and simple-viralizing (SV). Mutations that interfere with the active site—the heme group or residues involved in substrate binding—result in a complete loss of enzymatic activity, the salt-wasting type.[36] Studies have shown that mutations in the structure of 21-hydroxylase are related to the clinical severity of congenital adrenal hyperplasia.[37] Cortisol and aldosterone deficits are associated with life-threatening salt-loss (hence salt-wasting), as the steroids play roles in regulating sodium homeostasis. Retaining minimal enzyme activity, the simple-viralizing type is associated with mutations in conserved hydrophobic regions or near the transmembrane domain. Simple-viralizing CAH patients maintain adequate sodium homeostasis, but exhibit other phenotypical symptoms shared by SW, including accelerated growth in childhood and ambiguous genitalia in female neonates. Nonclassical forms retain 20-60% of hydroxylase function—this form is associated with normal cortisol expression, but an excess of androgens post-puberty.[38][39]
Non-classic congenital adrenal hyperplasia
Non-classical congenital adrenal hyperplasia caused by 21-hydroxylase deficiency (NCCAH) is a milder and late-onset congenital adrenal hyperplasia. Its prevalence rate in different ethnic groups varies from 1:1000 to 1:50.[13][40] Some people affected by the condition have no relevant signs and symptoms, while others experience symptoms of hyperandrogenism. Women with NCCAH usually have normal female genitalia at birth. In later life, the signs and symptoms of the condition in women may be different, but may include acne, hirsutism, male-pattern baldness, irregular menstruation, and infertility.[13][41][42] Fewer studies have been published about males with NCCAH comparing to those about females, because males are generally asymptomatic.[42][13] Males may have early beard growth and relatively small testes. Typically, they have normal sperm counts.[42]
References
- "Information on EC 1.14.14.16 - steroid 21-monooxygenase".
- Sumińska M, Bogusz-Górna K, Wegner D, Fichna M (June 2020). "Non-Classic Disorder of Adrenal Steroidogenesis and Clinical Dilemmas in 21-Hydroxylase Deficiency Combined with Backdoor Androgen Pathway. Mini-Review and Case Report". International Journal of Molecular Sciences. 21 (13): 4622. doi:10.3390/ijms21134622. PMC 7369945. PMID 32610579.
- Bergamaschi R, Livieri C, Uggetti C, Candeloro E, Egitto MG, Pichiecchio A, Cosi V, Bastianello S (March 2006). "Brain white matter impairment in congenital adrenal hyperplasia". Archives of Neurology. 63 (3): 413–6. doi:10.1001/archneur.63.3.413. PMID 16540460.
- Marcol W, Kalina-Faska B, Wackermann-Ramos A, Koehler B (2000). "Congenital adrenal hyperplasia conditioned by 21beta-hydroxylase deficiency - clinical considerations". Endokrynologia, Diabetologia I Choroby Przemiany Materii Wieku Rozwojowego (in Polish). 6 (1): 67–9. PMID 14640134.
- "NCBI: CYP21A2 cytochrome P450 family 21 subfamily A member 2". National Center for Biotechnology Information. Retrieved 30 November 2020.
This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This protein localizes to the endoplasmic reticulum and hydroxylates steroids at the 21 position. Its activity is required for the synthesis of steroid hormones including cortisol and aldosterone. Mutations in this gene cause congenital adrenal hyperplasia. A related pseudogene is located near this gene; gene conversion events involving the functional gene and the pseudogene are thought to account for many cases of steroid 21-hydroxylase deficiency. Two transcript variants encoding different isoforms have been found for this gene.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Ryan KJ, Engel LL (March 1957). "Hydroxylation of steroids at carbon 21" (PDF). The Journal of Biological Chemistry. 225 (1): 103–14. doi:10.1016/S0021-9258(18)64913-0. PMID 13416221.
- Pallan PS, Wang C, Lei L, Yoshimoto FK, Auchus RJ, Waterman MR, Guengerich FP, Egli M (May 2015). "Human Cytochrome P450 21A2, the Major Steroid 21-Hydroxylase: structure of the enzyme·progesterone substrate complex and rate-limiting c-h bond cleavage". The Journal of Biological Chemistry. 290 (21): 13128–43. doi:10.1074/jbc.M115.646307. PMC 4505568. PMID 25855791.
- Neunzig J, Milhim M, Schiffer L, Khatri Y, Zapp J, Sánchez-Guijo A, et al. (March 2017). "The steroid metabolite 16(β)-OH-androstenedione generated by CYP21A2 serves as a substrate for CYP19A1". The Journal of Steroid Biochemistry and Molecular Biology. 167: 182–191. doi:10.1016/j.jsbmb.2017.01.002. PMID 28065637. S2CID 36860068.
- Guengerich FP, Waterman MR, Egli M (August 2016). "Recent Structural Insights into Cytochrome P450 Function". Trends in Pharmacological Sciences. 37 (8): 625–40. doi:10.1016/j.tips.2016.05.006. PMC 4961565. PMID 27267697.
- Sushko TA, Gilep AA, Usanov SA (June 2012). "Mechanism of intermolecular interactions of microsomal cytochrome P450s CYP17 and CYP21 involved in steroid hormone biosynthesis". Biochemistry. Biokhimiia. 77 (6): 585–92. doi:10.1134/S0006297912060041. PMID 22817457. S2CID 18927484.
- Auchus RJ, Miller WL (2015). "P450 enzymes in steroid processing". Cytochrome P450: Structure, Mechanism, and Biochemistry (Fourth ed.). Springer International Publishing. pp. 851–879. doi:10.1007/978-3-319-12108-6_12. ISBN 978-3-319-12107-9.
- Araújo RS, Mendonca BB, Barbosa AS, Lin CJ, Marcondes JA, Billerbeck AE, Bachega TA (October 2007). "Microconversion between CYP21A2 and CYP21A1P promoter regions causes the nonclassical form of 21-hydroxylase deficiency". The Journal of Clinical Endocrinology and Metabolism. 92 (10): 4028–34. doi:10.1210/jc.2006-2163. PMID 17666484.
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. (November 2018). "Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline". The Journal of Clinical Endocrinology and Metabolism. 103 (11): 4043–4088. doi:10.1210/jc.2018-01865. PMC 6456929. PMID 30272171.
- Zhao B, Lei L, Kagawa N, Sundaramoorthy M, Banerjee S, Nagy LD, Guengerich FP, Waterman MR (March 2012). "Three-dimensional structure of steroid 21-hydroxylase (cytochrome P450 21A2) with two substrates reveals locations of disease-associated variants". The Journal of Biological Chemistry. 287 (13): 10613–22. doi:10.1074/jbc.M111.323501. PMC 3323056. PMID 22262854.
- Dorfman RI, Hayano M (March 1952). "The action of adrenal homogenates on progesterone, 17-hydroxyprogesterone and 21-desoxycortisone". Archives of Biochemistry and Biophysics. 36 (1): 237–9. doi:10.1016/0003-9861(52)90397-4. PMID 14934270.
- Auchus RJ, Sampath Kumar A, Andrew Boswell C, Gupta MK, Bruce K, Rath NP, Covey DF (January 2003). "The enantiomer of progesterone (ent-progesterone) is a competitive inhibitor of human cytochromes P450c17 and P450c21". Archives of Biochemistry and Biophysics. 409 (1): 134–44. doi:10.1016/s0003-9861(02)00491-5. PMID 12464252.
- Lorence MC, Trant JM, Mason JI, Bhasker CR, Fujii-Kuriyama Y, Estabrook RW, Waterman MR (August 1989). "Expression of a full-length cDNA encoding bovine adrenal cytochrome P450C21". Archives of Biochemistry and Biophysics. 273 (1): 79–88. doi:10.1016/0003-9861(89)90164-1. PMID 2502949.
- Wu DA, Hu MC, Chung BC (April 1991). "Expression and functional study of wild-type and mutant human cytochrome P450c21 in Saccharomyces cerevisiae". DNA and Cell Biology. 10 (3): 201–9. doi:10.1089/dna.1991.10.201. PMID 1707279.
- Graham SE, Peterson JA (2002). "Sequence alignments, variabilities, and vagaries". Methods in Enzymology. 357: 15–28. doi:10.1016/s0076-6879(02)57661-8. ISBN 9780121822606. PMID 12424893.
- ENSG00000231852, ENSG00000206338, ENSG00000233151, ENSG00000232414, ENSG00000235134 GRCh38: Ensembl release 89: ENSG00000198457, ENSG00000231852, ENSG00000206338, ENSG00000233151, ENSG00000232414, ENSG00000235134 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000024365 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Concolino P, Rizza R, Costella A, Carrozza C, Zuppi C, Capoluongo E (June 2017). "CYP21A2 intronic variants causing 21-hydroxylase deficiency". Metabolism: Clinical and Experimental. 71: 46–51. doi:10.1016/j.metabol.2017.03.003. PMID 28521877.
- Yu CY (1999). "Molecular genetics of the human MHC complement gene cluster". Experimental and Clinical Immunogenetics. 15 (4): 213–30. doi:10.1159/000019075. PMID 10072631. S2CID 25061446.
- White PC, Grossberger D, Onufer BJ, Chaplin DD, New MI, Dupont B, Strominger JL (February 1985). "Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man". Proceedings of the National Academy of Sciences of the United States of America. 82 (4): 1089–93. Bibcode:1985PNAS...82.1089W. doi:10.1073/pnas.82.4.1089. PMC 397199. PMID 2983330.
- Higashi Y, Yoshioka H, Yamane M, Gotoh O, Fujii-Kuriyama Y (May 1986). "Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene". Proceedings of the National Academy of Sciences of the United States of America. 83 (9): 2841–5. Bibcode:1986PNAS...83.2841H. doi:10.1073/pnas.83.9.2841. PMC 323402. PMID 3486422.
- Parker KL, Chaplin DD, Wong M, Seidman JG, Smith JA, Schimmer BP (December 1985). "Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells". Proceedings of the National Academy of Sciences of the United States of America. 82 (23): 7860–4. Bibcode:1985PNAS...82.7860P. doi:10.1073/pnas.82.23.7860. PMC 390869. PMID 2999780.
- Shiina T, Shimizu S, Hosomichi K, Kohara S, Watanabe S, Hanzawa K, et al. (June 2004). "Comparative genomic analysis of two avian (quail and chicken) MHC regions". Journal of Immunology. 172 (11): 6751–63. doi:10.4049/jimmunol.172.11.6751. PMID 15153492.
- Xie T, Rowen L, Aguado B, Ahearn ME, Madan A, Qin S, Campbell RD, Hood L (December 2003). "Analysis of the gene-dense major histocompatibility complex class III region and its comparison to mouse". Genome Research. 13 (12): 2621–36. doi:10.1101/gr.1736803. PMC 403804. PMID 14656967.
- Miller WL, Merke DP (2018). "Tenascin-X, Congenital Adrenal Hyperplasia, and the CAH-X Syndrome". Hormone Research in Paediatrics. 89 (5): 352–361. doi:10.1159/000481911. PMC 6057477. PMID 29734195.
- Milner CM, Campbell RD (August 2001). "Genetic organization of the human MHC class III region". Frontiers in Bioscience: A Journal and Virtual Library. 6: D914–26. doi:10.2741/milner. PMID 11487476.
- Espinosa Reyes TM, Collazo Mesa T, Lantigua Cruz PA, Agramonte Machado A, Domínguez Alonso E, Falhammar H (November 2020). "Molecular diagnosis of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency". BMC Endocrine Disorders. 20 (1): 165. doi:10.1186/s12902-020-00643-z. PMC 7653887. PMID 33168061.
- New MI, Wilson RC (October 1999). "Steroid disorders in children: congenital adrenal hyperplasia and apparent mineralocorticoid excess". Proceedings of the National Academy of Sciences of the United States of America. 96 (22): 12790–7. Bibcode:1999PNAS...9612790N. doi:10.1073/pnas.96.22.12790. PMC 23101. PMID 10536001.
- Therrell BL, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S (1998). "Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia". Pediatrics. 101 (4 Pt 1): 583–90. doi:10.1542/peds.101.4.583. PMID 9521938. S2CID 40355015.
- Pallan PS, Lei L, Wang C, Waterman MR, Guengerich FP, Egli M (September 2015). "Research Resource: Correlating Human Cytochrome P450 21A2 Crystal Structure and Phenotypes of Mutations in Congenital Adrenal Hyperplasia". Molecular Endocrinology. 29 (9): 1375–84. doi:10.1210/ME.2015-1127. PMC 4552440. PMID 26172259.
- Neves Cruz J, da Costa KS, de Carvalho TA, de Alencar NA (March 2020). "Measuring the structural impact of mutations on cytochrome P450 21A2, the major steroid 21-hydroxylase related to congenital adrenal hyperplasia". Journal of Biomolecular Structure & Dynamics. 38 (5): 1425–1434. doi:10.1080/07391102.2019.1607560. PMID 30982438. S2CID 115195169.
- Miller WL, Auchus RJ (February 2011). "The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders". Endocrine Reviews. 32 (1): 81–151. doi:10.1210/er.2010-0013. PMC 3365799. PMID 21051590.
- Haider S, Islam B, D'Atri V, Sgobba M, Poojari C, Sun L, Yuen T, Zaidi M, New MI (February 2013). "Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia". Proceedings of the National Academy of Sciences of the United States of America. 110 (7): 2605–10. Bibcode:2013PNAS..110.2605H. doi:10.1073/pnas.1221133110. PMC 3574933. PMID 23359706.
- Hannah-Shmouni F, Morissette R, Sinaii N, Elman M, Prezant TR, Chen W, et al. (November 2017). "Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi Jews and Caucasians". Genetics in Medicine. 19 (11): 1276–1279. doi:10.1038/gim.2017.46. PMC 5675788. PMID 28541281. S2CID 4630175.
- Merke DP, Auchus RJ (September 2020). "Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency". The New England Journal of Medicine. 383 (13): 1248–1261. doi:10.1056/NEJMra1909786. PMID 32966723.
- "Non-classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency".
External links
- GeneReviews/NCBI/NIH/UW entry on 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia
- OMIM entry on 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia
- Synthesis of Desoxycorticosterone from Progesterone through 21-Hydroxylase (Image)
- Steroid+21-Hydroxylase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human CPS1 genome location and CPS1 gene details page in the UCSC Genome Browser.
- Human CYP21A2 genome location and CYP21A2 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P08686 (Steroid 21-hydroxylase) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.