Prenderol
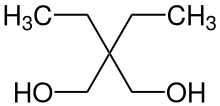
Prenderol (Diethylpropanediol) is a simple alkyl diol which has sedative, anticonvulsant and muscle relaxant effects. It is closely related in structure to meprobamate and numerous other alkyl alcohols and diols with generally comparable activity.[1][2][3][4][5][6]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.003.731 |
| Chemical and physical data | |
| Formula | C7H16O2 |
| Molar mass | 132.203 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 59–62 °C (138–144 °F) |
| |
| |
See also
References
- Berger FM (June 1949). "Anticonvulsant action of 2-substituted-1,3-propanediols". Proceedings of the Society for Experimental Biology and Medicine. New York, N.Y. 71 (2): 270–271. doi:10.3181/00379727-71-17159. PMID 18134033. S2CID 32255832.
- Funderburk WH, Unna KR (March 1953). "Site of action of 2, 2-diethyl-1,3-propanediol (prenderol) on the central nervous system". The Journal of Pharmacology and Experimental Therapeutics. 107 (3): 344–55. PMID 13035673.
- Mailer AB (July 1954). "Effects of mephenesin and prenderol on intellectual functions of mental patients". Journal of Clinical Psychology. 10 (3): 283–5. doi:10.1002/1097-4679(195407)10:3<283::aid-jclp2270100322>3.0.co;2-a. PMID 13163224.
- Blum B (May 1962). "Head shaking and nystagmus produced by 2,2-diethyl, 1,3-propanediol (prenderol) in the rat". Archives Internationales de Pharmacodynamie et de Therapie. 137: 128–36. PMID 13870189.
- Reyes Q., Aaurelio; Mascetti V., G.; Martinez J., Rolando. Synthesis of polyhydroxylated alcohols. Revista Latinoamericana de Quimica 1984; 15 (1): 29-30. ISSN: 0370-5943.
- Wang G, Lu Q (October 2013). "A nitrate ester of sedative alkyl alcohol improves muscle function and structure in a murine model of Duchenne muscular dystrophy". Molecular Pharmaceutics. 10 (10): 3862–70. doi:10.1021/mp400310r. PMID 23924275.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
