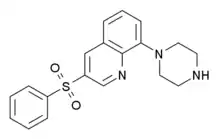
Intepirdine
Intepirdine (INN; developmental codes SB-742457, RVT-101)[1] is a selective 5-HT6 receptor antagonist with potential cognition, memory, and learning-enhancing effects.[2][3] It was under development by GlaxoSmithKline for the treatment of Alzheimer's disease and demonstrated some preliminary efficacy in phase II clinical trials.[3] GSK chose not to continue development and sold the rights to Axovant Sciences for $5 million in December 2014.[4]
 | |
| Clinical data | |
|---|---|
| Other names | SB-742457, RVT-101 |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.094 |
| Chemical and physical data | |
| Formula | C19H19N3O2S |
| Molar mass | 353.44 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Results of a phase III clinical trial for the treatment of Alzheimer's disease were reported in September 2017.[5] The trial showed no improvement over control group and Axovant lost 70% of its value upon the announcement of the trial results.[6]
Intepirdine also entered clinical trials for dementia with Lewy bodies, also with negative results.[7] Consequently, Axovant announced in 2018 that it has discontinued development of this drug.[7]
References
- "Intepirdine - Axovant Sciences / GlaxoSmithKline". AdisInsight. Retrieved 9 January 2018.
- Upton N, Chuang TT, Hunter AJ, Virley DJ (July 2008). "5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer's disease". Neurotherapeutics. 5 (3): 458–69. doi:10.1016/j.nurt.2008.05.008. PMC 5084247. PMID 18625457.
- Rossé G, Schaffhauser H (February 2010). "5-HT(6) Receptor Antagonists as Potential Therapeutics for Cognitive Impairment". Current Topics in Medicinal Chemistry. 10 (2): 207–21. doi:10.2174/156802610790411036. PMID 20166958.
- Lowe, Derek (12 May 2015). "An Alzheimer's IPO, Because Why Not". In the Pipeline.
- Fiore, Kristina (27 September 2016). "NeuroBreak: Alzheimer's Drug Bombs; Elephant Tranquilizer ODs". MedPage Today.
- Garde, Damian (26 September 2017). "Another Alzheimer's failure: Axovant's drug flops in late-stage trial". STAT.
- Taylor, Phil (Jan 8, 2018). "Axovant slumps as it dumps lead drug intepirdine". Fierce Biotech.