Sunepitron
Sunepitron (CP-93,393) is a combined 5-HT1A receptor agonist and α2-adrenergic receptor antagonist.[1][2] It was previously under development by Pfizer for the treatment of depression and anxiety.[3] It made it to phase III clinical trials before being discontinued.[2][3]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H23N5O2 |
| Molar mass | 329.404 g·mol−1 |
| 3D model (JSmol) | |
| |
Synthesis
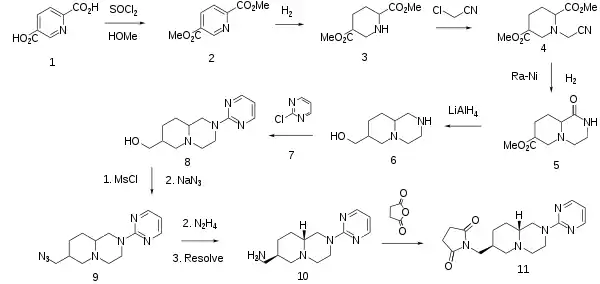
The synthesis starts by conversion of the pyridine dicarboxylic acid (1) to its acid chloride; rxn with MeOH then affords the ester (2). Catalytic hydrogenation serves to reduce the pyridine ring to a piperidine of undefined stereochemistry (3). Alkylation of this intermediate with chloroacetonitrile affords (4). Treatment of that intermediate with Raney nickel reduces the cyano group to the corresponding primary amine; this product then undergoes an internal ester-amine interchange to yield the cyclized lactam (5). LAH serves to reduce the lactam to an amine; the ester on the other ring is reduced to a carbinol in the process, affording the aminoalcohol (7). The basic function is next alkylated with 2-chloropyrimidine (7). Rxn of the alcoholin (8) with MsCl leads to the mesylate; that group is next displaced by sodium azide (9); the azide group is next reduced to the primary amine. Resolution of this product as its mandelate salt then yields (10) as a single enantiomer. Rxn of that product with succinic anhydride converts the pendant amine to a succinimide, affording the anxiolytic agent sunepitron (1).
See also
References
- Goodnick PJ (July 1999). "Psychopharmacology of depression in the next millennium". CNS Spectrums. 4 (7): 21–35. doi:10.1017/s1092852900011998. PMID 18438295.
- Stahl SM (2000). Essential psychopharmacology: neuroscientific basis and practical application. Cambridge, UK: Cambridge University Press. p. 265. ISBN 0-521-64615-4.
- Kaplan EP, Turkington C (2001). Making the antidepressant decision: how to choose the right treatment option for you and your loved ones. Chicago, Ill: Contemporary Books. ISBN 0-7373-0417-0.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| α1 |
| ||||
|---|---|---|---|---|---|
| α2 |
| ||||
| β |
| ||||
| See also | |||||
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||