Quipazine
Quipazine is a piperazine drug used in scientific research, originally intended as an antidepressant but never developed for medical use. It is a serotonin reuptake inhibitor,[1] and also a moderately selective serotonin receptor agonist, binding to a range of different serotonin receptors, but particularly to the 5-HT2A[2] and 5-HT3 subtypes.[3][4] It produces a head-twitch response in animal studies,[5] but failed to produce psychedelic effects in humans at a dose of 25mg, which was the highest dose tested due to 5-HT3 mediated side effects of nausea and gastrointestinal discomfort.[6] However Alexander Shulgin claimed that an effective psychedelic dose could be reached by blocking 5-HT3 receptors using a 5-HT3 antagonist.[7]
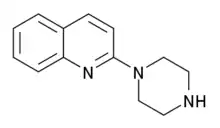 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.885 |
| Chemical and physical data | |
| Formula | C13H15N3 |
| Molar mass | 213.284 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Cappelli A, Giuliani G, Gallelli A, Valenti S, Anzini M, Mennuni L, Makovec F, Cupello A, Vomero S. Structure-affinity relationship studies on arylpiperazine derivatives related to quipazine as serotonin transporter ligands. Molecular basis of the selectivity SERT/5HT3 receptor. Bioorg Med Chem. 2005 May 16;13(10):3455-60. doi:10.1016/j.bmc.2005.03.008 PMID 15848758
- Smith RL, Barrett RJ, Sanders-Bush E (November 1995). "Neurochemical and behavioral evidence that quipazine-ketanserin discrimination is mediated by serotonin2A receptor". The Journal of Pharmacology and Experimental Therapeutics. 275 (2): 1050–7. PMID 7473132.
- Cappelli A, Anzini M, Vomero S, Mennuni L, Makovec F, Doucet E, et al. (February 1998). "Novel potent and selective central 5-HT3 receptor ligands provided with different intrinsic efficacy. 1. Mapping the central 5-HT3 receptor binding site by arylpiperazine derivatives". Journal of Medicinal Chemistry. 41 (5): 728–41. doi:10.1021/jm970645i. PMID 9513601.
- Cappelli A, Butini S, Brizzi A, Gemma S, Valenti S, Giuliani G, et al. (2010). "The interactions of the 5-HT3 receptor with quipazine-like arylpiperazine ligands: the journey track at the end of the first decade of the third millennium". Curr Top Med Chem. 10 (5): 504–26. doi:10.2174/156802610791111560. PMID 20166948.
- de la Fuente Revenga M, Shah UH, Nassehi N, Jaster AM, Hemanth P, Sierra S, Dukat M, González-Maeso J (January 2021). "Psychedelic-like Properties of Quipazine and Its Structural Analogues in Mice". ACS Chemical Neuroscience. doi:10.1021/acschemneuro.0c00291. PMID 33400504.
- Winter JC. "The stimulus effects of serotonergic hallucinogens in animals". NIDA Research Monograph. 146: 157–82. PMID 8742798.
- Halberstadt AL, Geyer MA. "Effect of Hallucinogens on Unconditioned Behavior". Current Topics in Behavioral Neurosciences. 36: 159–199. doi:10.1007/7854_2016_466. PMC 5787039. PMID 28224459.
- DE 2006638, Rodriguez R, issued 1970 Chem. Abstr., 73: 98987g (1970).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.