Roluperidone
Roluperidone (former developmental code names MIN-101, CYR-101, MT-210) is a 5-HT2A and σ2 receptor antagonist that is under development by Minerva Neurosciences for the treatment of schizophrenia.[1][2][3][4] One of its metabolites also has some affinity for the H1 receptor.[2] As of May 2018, the drug is in phase III clinical trials.[5]
 | |
| Clinical data | |
|---|---|
| Other names | MIN-101; CYR-101; MT-210 |
| Routes of administration | By mouth |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
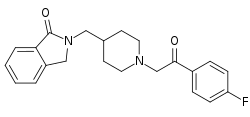
| Formula | C22H23F2N2O2 |
| Molar mass | 385.435 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Mestre TA, Zurowski M, Fox SH (April 2013). "5-Hydroxytryptamine 2A receptor antagonists as potential treatment for psychiatric disorders". Expert Opinion on Investigational Drugs. 22 (4): 411–21. doi:10.1517/13543784.2013.769957. PMID 23409724.
- Ebdrup BH, Rasmussen H, Arnt J, Glenthøj B (September 2011). "Serotonin 2A receptor antagonists for treatment of schizophrenia". Expert Opinion on Investigational Drugs. 20 (9): 1211–23. doi:10.1517/13543784.2011.601738. PMID 21740279.
- Köster LS, Carbon M, Correll CU (December 2014). "Emerging drugs for schizophrenia: an update". Expert Opinion on Emerging Drugs. 19 (4): 511–31. doi:10.1517/14728214.2014.958148. PMID 25234340.
- "Drug Development in Schizophrenia: Summary and Table". Pharmaceutical Medicine. 28 (5): 265–271. 2014. doi:10.1007/s40290-014-0070-6. ISSN 1178-2595.
- "Roluperidone - Minerva Neurosciences". Adis Insight. Springer Nature Switzerland AG.
| σ1 |
|
|---|---|
| σ2 |
|
| Unsorted |
|
See also: Receptor/signaling modulators | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.