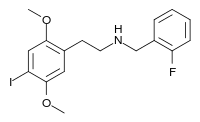
25I-NBF
25I-NBF (2C-I-NBF, NBF-2C-I, Cimbi-21) is a derivative of the phenethylamine hallucinogen 2C-I, which acts as a highly potent partial agonist for the human 5-HT2A receptor.[1][2][3] It has been studied in its 11C radiolabelled form as a potential ligand for mapping the distribution of 5-HT2A receptors in the brain, using positron emission tomography (PET).[4][5]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H19FINO2 |
| Molar mass | 415.247 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Legality
25I-NBF is illegal in Hungary,[6] Japan,[7] Latvia,[8] and Vermont.[9]
Sweden
The Riksdag added 25I-NBF to Narcotic Drugs Punishments Act under swedish schedule I ("substances, plant materials and fungi which normally do not have medical use") as of January 26, 2016, published by Medical Products Agency (MPA) in regulation HSLF-FS 2015:35 listed as 25I-NBF, and 2-(4-jodo-2,5-dimetoxifenyl)-N-(2-fluorobensyl)etanamin.[10]
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[11]
Analogues and derivatives
Analogues and derivatives of 2C-I:
25I-NB*:
- 25I-NBF
- 25I-NBMD
- 25I-NB34MD
- 25I-NBOH
- 25I-NBOMe (NBOMe-2CI)
- 25I-NB3OMe
- 25I-NB4OMe
References
- Braden MR, Parrish JC, Naylor JC, Nichols DE (December 2006). "Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists". Molecular Pharmacology. 70 (6): 1956–64. doi:10.1124/mol.106.028720. PMID 17000863. S2CID 15840304.
- Braden MR (2007). Towards a biophysical understanding of hallucinogen action (Ph.D. thesis). Purdue University. ProQuest 304838368.
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL (March 2014). "Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists". ACS Chemical Neuroscience. 5 (3): 243–9. doi:10.1021/cn400216u. PMC 3963123. PMID 24397362.
- Hansen M (2010-12-16). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (Ph.D. thesis). University of Copenhagen. doi:10.13140/RG.2.2.33671.14245.
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–93. doi:10.1007/s00259-010-1686-8. PMID 21174090.
- "A Magyarországon megjelent, a Kábítószer és Kábítószer-függőség Európai Megfigyelő Központjának Korai Jelzőrendszerébe (EMCDDA EWS) 2005 óta bejelentett ellenőrzött anyagok büntetőjogi vonatkozású besorolása" [Criminal classification of controlled substances published in Hungary and notified to the European Monitoring Center for Drugs and Drug Addiction (EMCDDA EWS) since 2005] (PDF) (in Hungarian).
- "指定薬物名称・構造式一覧(平成27年9月16日現在)" (PDF) (in Japanese). 厚生労働省. 16 September 2015. Retrieved 8 October 2015.
- "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem" [Regulations on Narcotic Drugs, Psychotropic Substances and Precursors Controlled in Latvia]. Ministry of Health of the Republic of Latvia (in Latvian).
- "Regulated Drugs Rule" (PDF). Vermont Department of Health. Retrieved 14 October 2015.
- Ödman P. "Gemensamma författningssamlingen avseende hälso- och sjukvård, socialtjänst, läkemedel, folkhälsa m.m." [Joint constitutional collection on health care, social services, pharmaceuticals, public health, etc.] (PDF). Lakemedelsverket (in Swedish).
- "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". www.legislation.gov.uk.