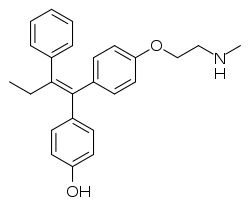
Endoxifen
Endoxifen, also known as 4-hydroxy-N-desmethyltamoxifen, is a chemical that is under development for estrogen receptor-positive breast cancer.[1] It is also being evaluated as an antipsychotic for treatment of mania and other psychotic disorders.[2]
 | |
| Clinical data | |
|---|---|
| Other names | 4-Hydroxy-N-desmethyltamoxifen; Desmethylhydroxytamoxifen |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| UNII | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.208.548 |
| Chemical and physical data | |
| Formula | C25H27NO2 |
| Molar mass | 373.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Endoxifen is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group. It is an active metabolite of tamoxifen and has been found to be effective in patients that have failed previous hormonal therapies (tamoxifen, aromatase inhibitors, and fulvestrant).[3][4][5] The prodrug tamoxifen is metabolized by the CYP2D6 enzyme to produce endoxifen and afimoxifene (4-hydroxytamoxifen).[6]
In the first study to evaluate the pharmacology of endoxifen, it showed 25% of the affinity of estradiol for the estrogen receptor (ER) while afimoxifene had 35% of the affinity of estradiol for the ER.[7] The antiestrogenic actions of endoxifen and afimoxifene in this study were very similar.[7] In another study, the affinity of endoxifen for the ERα was 12.1% and its affinity for the ERβ was 4.75% relative to estradiol.[8] For comparison, afimoxifene had relative binding affinities for the ERα and ERβ of 19.0% and 21.5% compared to estradiol, respectively.[8] In yet another investigation, both endoxifen and afimoxifene had 181% of the affinity of estradiol for the ER whereas tamoxifen had 2.8% and N-desmethyltamoxifen had 2.4%.[9]
See also
- List of investigational hormonal agents § Estrogenics
- Droloxifene (3-hydroxytamoxifen)
- Norendoxifen (4-hydroxy-N-didesmethyltamoxifen)
References
- "Z-endoxifen hydrochloride". NCI Drug Dictionary.
- Rankovic Z, Bingham M, Hargreaves R, eds. (2012). Drug Discovery for Psychiatric Disorders. Royal Society of Chemistry. p. 349. ISBN 978-1-84973-365-6.
- Hawse JR, Subramaniam M, Cicek M, Wu X, Gingery A, Grygo SB, Sun Z, Pitel KS, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC (2013). "Endoxifen's molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens". PLOS ONE. 8 (1): e54613. Bibcode:2013PLoSO...854613H. doi:10.1371/journal.pone.0054613. PMC 3557294. PMID 23382923. Lay summary – Medical Daily (December 12, 2013).
- Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC (March 2009). "The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells". Cancer Research. 69 (5): 1722–7. doi:10.1158/0008-5472.CAN-08-3933. PMID 19244106.
- Gingery A, Subramaniam M, Pitel KS, Reese JM, Cicek M, Lindenmaier LB, Ingle JN, Goetz MP, Turner RT, Iwaniec UT, Spelsberg TC, Hawse JR (2014). "The effects of a novel hormonal breast cancer therapy, endoxifen, on the mouse skeleton". PLOS ONE. 9 (5): e98219. Bibcode:2014PLoSO...998219G. doi:10.1371/journal.pone.0098219. PMC 4031133. PMID 24853369.
- Wilcken N (2016). "Breast cancer: a disease of subtypes". Cancer Forum. 40 (3).
- Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC (May 2004). "Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen". Breast Cancer Res Treat. 85 (2): 151–9. doi:10.1023/B:BREA.0000025406.31193.e8. hdl:2027.42/44223. PMID 15111773.
- Kelly PM, Keely NO, Bright SA, Yassin B, Ana G, Fayne D, Zisterer DM, Meegan MJ (August 2017). "Novel Selective Estrogen Receptor Ligand Conjugates Incorporating Endoxifen-Combretastatin and Cyclofenil-Combretastatin Hybrid Scaffolds: Synthesis and Biochemical Evaluation". Molecules. 22 (9). doi:10.3390/molecules22091440. PMC 6151695. PMID 28858267.
- Maximov PY, McDaniel RE, Fernandes DJ, Bhatta P, Korostyshevskiy VR, Curpan RF, Jordan VC (October 2014). "Pharmacological relevance of endoxifen in a laboratory simulation of breast cancer in postmenopausal patients". J Natl Cancer Inst. 106 (10). doi:10.1093/jnci/dju283. PMC 4271116. PMID 25258390.