Pentolame
Pentolame, also known as 17β-((5-hydroxypentyl)amino)estradiol is a synthetic, steroidal estrogen and a 17β-aminoestrogen with anticoagulant effects that was first described in 1993 and was never marketed.[1][2][3]
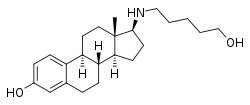 | |
| Clinical data | |
|---|---|
| Other names | 17β-((5-Hydroxypentyl)amino)estradiol; 17β-[(5-Hydroxypentyl)amino]estra-1,3,5(10)-trien-3-ol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C23H35NO2 |
| Molar mass | 357.538 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Lemini C, Rubio-Póo C, Silva G, García-Mondragón J, Zavala E, Mendoza-Patiño N, Castro D, Cruz-Almanza R, Mandoki JJ (1993). "Anticoagulant and estrogenic effects of two new 17 beta-aminoestrogens, butolame [17 beta-(4-hydroxy-1-butylamino)-1,3,5(10)-estratrien-3-ol] and pentolame [17 beta-(5-hydroxy-1-pentylamino)-1,3,5(10)-estratrien-3-ol]". Steroids. 58 (10): 457–61. doi:10.1016/0039-128x(93)90002-5. PMID 8256254. S2CID 54381037.
- Lemus AE, Jaimez R, Lemini C, Menjivar M, Silva G, Rubio-Poo C, Valenzuela F, Larrea F (1998). "Estrogenic effects of the synthetic aminoestrogen 17 beta-(5-hydroxy-1-pentylamino)-1,3,5(10)-estratrien-3-ol (pentolame)". Steroids. 63 (7–8): 433–8. doi:10.1016/s0039-128x(98)00046-4. PMID 9654651. S2CID 13582499.
- Jaimez R, Cooney A, Jackson K, Lemus AE, Lemini C, Cárdenas M, García R, Silva G, Larrea F (2000). "In vivo estrogen bioactivities and in vitro estrogen receptor binding and transcriptional activities of anticoagulant synthetic 17beta-aminoestrogens". J. Steroid Biochem. Mol. Biol. 73 (1–2): 59–66. doi:10.1016/s0960-0760(00)00053-4. PMID 10822025. S2CID 40211307.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.