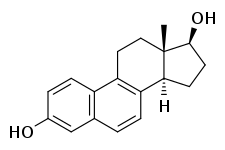
17β-Dihydroequilenin
17β-Dihydroequilenin, or β-dihydroequilenin, also known as δ6,8-17β-estradiol or 6,8-didehydro-17β-estradiol, as well as estra-1,3,5(10),6,8-pentaen-3,17β-diol, is a naturally occurring steroidal estrogen found in horses which is closely related to equilin, equilenin, and estradiol, and, as the 3-sulfate ester sodium salt, is a minor constituent (0.5%) of conjugated estrogens (Premarin).[1] 17β-Dihydroequilenin has unexpectedly shown a selective estrogen receptor modulator (SERM)-like profile of estrogenic activity in studies with monkeys, in which beneficial effects on bone and the cardiovascular system were noted but proliferative responses in breast and endometrium were not observed.[2]
 | |
| Clinical data | |
|---|---|
| Other names | β-Dihydroequilenin; Δ6,8-17β-Estradiol; 6,8-Didehydro-17β-estradiol; Estra-1,3,5(10),6,8-pentaen-3,17β-diol |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H20O2 |
| Molar mass | 268.356 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 751–. ISBN 978-1-4511-4847-3.
- Cline JM (2007). "Assessing the mammary gland of nonhuman primates: effects of endogenous hormones and exogenous hormonal agents and growth factors". Birth Defects Research Part B: Developmental and Reproductive Toxicology. 80 (2): 126–46. doi:10.1002/bdrb.20112. PMID 17443713.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.