Taste receptor
A taste receptor is a type of receptor which facilitates the sensation of taste. When food or other substances enter the mouth, molecules interact with saliva and are bound to taste receptors in the oral cavity and other locations. Molecules which give a sensation of taste are considered "sapid".[1]
| Taste receptor | |
|---|---|
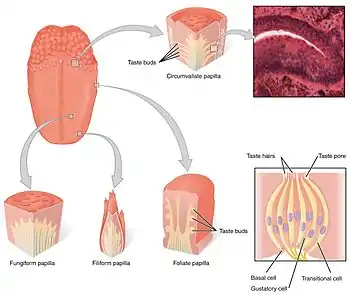 Taste receptors of the tongue are present in the taste buds of papillae. | |
| Identifiers | |
| FMA | 84662 |
| Anatomical terminology | |
Vertebrate taste receptors are divided into two families:
- Type 1, sweet, first characterized in 2001:[2] TAS1R2 – TAS1R3
- Type 2, bitter, first characterized in 2000:[3] In humans there are 25 known different bitter receptors, in cats there are 12, in chickens there are three, and in mice there are 35 known different bitter receptors.[4]
Visual, olfactive, "sapictive" (the perception of tastes), trigeminal (hot, cool), mechanical, all contribute to the perception of taste. Of these, transient receptor potential cation channel subfamily V member 1 (TRPV1) vanilloid receptors are responsible for the perception of heat from some molecules such as capsaicin, and a CMR1 receptor is responsible for the perception of cold from molecules such as menthol, eucalyptol, and icilin.[1]
Tissue distribution
The gustatory system consists of taste receptor cells in taste buds. Taste buds, in turn, are contained in structures called papillae. There are three types of papillae involved in taste: fungiform papillae, foliate papillae, and circumvallate papillae. (The fourth type - filiform papillae do not contain taste buds). Beyond the papillae, taste receptors are also in the palate and early parts of the digestive system like the larynx and upper esophagus. There are three cranial nerves that innervate the tongue; the vagus nerve, glossopharyngeal nerve, and the facial nerve. The glossopharyngeal nerve and the chorda tympani branch of the facial nerve innervate the TAS1R and TAS2R taste receptors. Next to the taste receptors in on the tongue, the gut epithelium is also equipped with a subtle chemosensory system that communicates the sensory information to several effector systems involved in the regulation of appetite, immune responses, and gastrointestinal motility [5]
In 2010, researchers found bitter receptors in lung tissue, which cause airways to relax when a bitter substance is encountered. They believe this mechanism is evolutionarily adaptive because it helps clear lung infections, but could also be exploited to treat asthma and chronic obstructive pulmonary disease.[6]
Function
Taste helps to identify toxins, maintain nutrition, and regulate appetite, immune responses, and gastrointestinal motility.[5] Five basic tastes are recognized today: salty, sweet, bitter, sour, and umami. Salty and sour taste sensations are both detected through ion channels. Sweet, bitter, and umami tastes, however, are detected by way of G protein-coupled taste receptors.[7]
In addition, some agents can function as taste modifiers, as miraculin or curculin for sweet or sterubin to mask bitter.
Mechanism of action
The standard bitter, sweet, or umami taste receptor is a G protein-coupled receptor with seven transmembrane domains. Ligand binding at the taste receptors activate second messenger cascades to depolarize the taste cell. Gustducin is the most common taste Gα subunit, having a major role in TAS2R bitter taste reception. Gustducin is a homologue for transducin, a G-protein involved in vision transduction.[8] Additionally, taste receptors share the use of the TRPM5 ion channel, as well as a phospholipase PLCβ2.[9]
Savory or glutamates (Umami)
The TAS1R1+TAS1R3 heterodimer receptor functions as an umami receptor, responding to L-amino acid binding, especially L-glutamate.[2] The umami taste is most frequently associated with the food additive monosodium glutamate (MSG) and can be enhanced through the binding of inosine monophosphate (IMP) and guanosine monophosphate (GMP) molecules.[10][11] TAS1R1+3 expressing cells are found mostly in the fungiform papillae at the tip and edges of the tongue and palate taste receptor cells in the roof of the mouth.[2] These cells are shown to synapse upon the chorda tympani nerves to send their signals to the brain, although some activation of the glossopharyngeal nerve has been found.[10][12]
Alternative candidate umami taste receptors include splice variants of metabotropic glutamate receptors, mGluR4 and mGluR1, and the NMDA receptor.[7][13][14][15]
Sweet

The TAS1R2+TAS1R3 heterodimer receptor functions as the sweet receptor by binding to a wide variety of sugars and sugar substitutes.[2][16] TAS1R2+3 expressing cells are found in circumvallate papillae and foliate papillae near the back of the tongue and palate taste receptor cells in the roof of the mouth.[2] These cells are shown to synapse upon the chorda tympani and glossopharyngeal nerves to send their signals to the brain.[7][12] The TAS1R3 homodimer also functions as a sweet receptor in much the same way as TAS1R2+3 but has decreased sensitivity to sweet substances. Natural sugars are more easily detected by the TAS1R3 receptor than sugar substitutes. This may help explain why sugar and artificial sweeteners have different tastes.[17] Genetic polymorphisms in TAS1R3 partly explain the difference in sweet taste perception and sugar consumption between people of African American ancestry and people of European and Asian ancestries.[18][19]
Bitter
The TAS2R proteins (InterPro: IPR007960) function as bitter taste receptors.[20] There are 43 human TAS2R genes, each of which (excluding the five pseudogenes) lacks introns and codes for a GPCR protein.[7] These proteins, as opposed to TAS1R proteins, have short extracellular domains and are located in circumvallate papillae, palate, foliate papillae, and epiglottis taste buds, with reduced expression in fungiform papillae.[3][7] Though it is certain that multiple TAS2Rs are expressed in one taste receptor cell, it is still debated whether mammals can distinguish between the tastes of different bitter ligands.[3][7] Some overlap must occur, however, as there are far more bitter compounds than there are TAS2R genes. Common bitter ligands include cycloheximide, denatonium, PROP (6-n-propyl-2-thiouracil), PTC (phenylthiocarbamide), and β-glucopyranosides.[7]
Signal transduction of bitter stimuli is accomplished via the α-subunit of gustducin. This G protein subunit activates a taste phosphodiesterase and decreases cyclic nucleotide levels. Further steps in the transduction pathway are still unknown. The βγ-subunit of gustducin also mediates taste by activating IP3 (inositol triphosphate) and DAG (diglyceride). These second messengers may open gated ion channels or may cause release of internal calcium.[21] Though all TAS2Rs are located in gustducin-containing cells, knockout of gustducin does not completely abolish sensitivity to bitter compounds, suggesting a redundant mechanism for bitter tasting[9] (unsurprising given that a bitter taste generally signals the presence of a toxin).[9] One proposed mechanism for gustducin-independent bitter tasting is via ion channel interaction by specific bitter ligands, similar to the ion channel interaction which occurs in the tasting of sour and salty stimuli.[7]
One of the best-researched TAS2R proteins is TAS2R38, which contributes to the tasting of both PROP and PTC. It is the first taste receptor whose polymorphisms are shown to be responsible for differences in taste perception. Current studies are focused on determining other such taste phenotype-determining polymorphisms.[7] More recent studies show that genetic polymorphisms in other bitter taste receptor genes influence bitter taste perception of caffeine, quinine and denatonium benzoate.[22]

Sour
Historically it was thought that the sour taste was produced solely when free hydrogen ions (H+) directly depolarised taste receptors. However, specific receptors for sour taste with other methods of action are now being proposed. The HCN channels were such a proposal; as they are cyclic nucleotide-gated channels. The two ion channels now suggested to contribute to sour taste are ASIC2 and TASK-1.

Salt
Various receptors have also been proposed for salty tastes, along with the possible taste detection of lipids, complex carbohydrates, and water. Evidence for these receptors is, however, shaky at best, and is often unconvincing in mammal studies. For example, the proposed ENaC receptor for sodium detection can only be shown to contribute to sodium taste in Drosophilia.[7]
Carbonation
An enzyme connected to the sour receptor transmits information about carbonated water.[23]
Fat
A possible taste receptor for fat, CD36, has been identified.[24] CD36 has been localized to the circumvallate and foliate papillae, which are present in taste buds[25] and where lingual lipase is produced, and research has shown that the CD36 receptor binds long chain fatty acids.[26] Differences in the amount of CD36 expression in human subjects was associated with their ability to taste fats,[27] creating a case for the receptor's relationship to fat tasting. Further research into the CD36 receptor could be useful in determining the existence of a true fat-tasting receptor.
GPR120 and GPR40 have been implicated to respond to oral fat,[28] and their absence leads to reduced fat preference and reduced neuronal response to orally administered fatty acids.[29]
TRPM5 has been shown to be involved in oral fat response and identified as a possible oral fat receptor, but recent evidence presents it as primarily a downstream actor.[30][31]
Types
Human bitter taste receptor genes are named TAS2R1 to TAS2R64, with many gaps due to non-existent genes, pseudogenes or proposed genes that have not been annotated to the most recent human genome assembly. Many bitter taste receptor genes also have confusing synonym names with several different gene names referring to the same gene. See table below for full list of human bitter taste receptor genes:
| Class | Gene | Synonyms | Aliases | Locus | Description |
|---|---|---|---|---|---|
| type 1 (sweet) |
TAS1R1 | GPR70 | 1p36.23 | ||
| TAS1R2 | GPR71 | 1p36.23 | |||
| TAS1R3 | 1p36 | ||||
| type 2 (bitter) |
TAS2R1 | 5p15 | |||
| TAS2R2 | 7p21.3 | pseudogene | |||
| TAS2R3 | 7q31.3-q32 | ||||
| TAS2R4 | 7q31.3-q32 | ||||
| TAS2R5 | 7q31.3-q32 | ||||
| TAS2R6 | 7 | not annotated in human genome assembly | |||
| TAS2R7 | 12p13 | ||||
| TAS2R8 | 12p13 | ||||
| TAS2R9 | 12p13 | ||||
| TAS2R10 | 12p13 | ||||
| TAS2R11 | absent in humans | ||||
| TAS2R12 | TAS2R26 | 12p13.2 | pseudogene | ||
| TAS2R13 | 12p13 | ||||
| TAS2R14 | 12p13 | ||||
| TAS2R15 | 12p13.2 | pseudogene | |||
| TAS2R16 | 7q31.1-q31.3 | ||||
| TAS2R17 | absent in humans | ||||
| TAS2R18 | 12p13.2 | pseudogene | |||
| TAS2R19 | TAS2R23, TAS2R48 | 12p13.2 | |||
| TAS2R20 | TAS2R49 | 12p13.2 | |||
| TAS2R21 | absent in humans | ||||
| TAS2R22 | 12 | not annotated in human genome assembly | |||
| TAS2R24 | absent in humans | ||||
| TAS2R25 | absent in humans | ||||
| TAS2R27 | absent in humans | ||||
| TAS2R28 | absent in humans | ||||
| TAS2R29 | absent in humans | ||||
| TAS2R30 | TAS2R47 | 12p13.2 | |||
| TAS2R31 | TAS2R44 | 12p13.2 | |||
| TAS2R32 | absent in humans | ||||
| TAS2R33 | 12 | not annotated in human genome assembly | |||
| TAS2R34 | absent in humans | ||||
| TAS2R35 | absent in humans | ||||
| TAS2R36 | 12 | not annotated in human genome assembly | |||
| TAS2R37 | 12 | not annotated in human genome assembly | |||
| TAS2R38 | 7q34 | ||||
| TAS2R39 | 7q34 | ||||
| TAS2R40 | GPR60 | 7q34 | |||
| TAS2R41 | 7q34 | ||||
| TAS2R42 | 12p13 | ||||
| TAS2R43 | 12p13.2 | ||||
| TAS2R45 | GPR59 | 12 | |||
| TAS2R46 | 12p13.2 | ||||
| TAS2R50 | TAS2R51 | 12p13.2 | |||
| TAS2R52 | absent in humans | ||||
| TAS2R53 | absent in humans | ||||
| TAS2R54 | absent in humans | ||||
| TAS2R55 | absent in humans | ||||
| TAS2R56 | absent in humans | ||||
| TAS2R57 | absent in humans | ||||
| TAS2R58 | absent in humans | ||||
| TAS2R59 | absent in humans | ||||
| TAS2R60 | 7 | ||||
| TAS2R62P | 7q34 | pseudogene | |||
| TAS2R63P | 12p13.2 | pseudogene | |||
| TAS2R64P | 12p13.2 | pseudogene |
Loss of function
In many species, taste receptors have shown loss of functions. The evolutionary process of which taste receptors lost their function is believed to be an adaptive evolution where it is associated with feeding ecology to drive specialization and bifurcation of taste receptors.[32] Out of all the taste receptors, bitter, sweet, and umami are shown to have a correlation between inactivation of taste receptors and feeding behavior.[32] However, there are no strong evidences that support any vertebrates are missing the bitter taste receptor genes.[32]
The sweet taste receptor is one of the taste receptors where the function has been lost. In mammals, the predominant sweet taste receptor is the Type 1 taste receptor Tas1r2/Tas1r3.[33] Some mammalian species such as cats and vampire bats have shown inability to taste sweet.[33] In these species, the cause of loss of function of the sweet receptor is due to the pseudogenization of Tas1r2.[33] The pseudogenization of Tas1r2 is also observed in non-mammalian species such as chickens and tongueless Western clawed frog, and these species also show the inability to taste sweet.[33] The pseudogenization of Tas1r2 is widespread and independent in the order Carnivora.[33] Many studies have shown that the pseudogenization of taste receptors is caused by a deleterious mutation in the open reading frames (ORF).[34] In a study, it was found that in nonfeline carnivorous species, these species showed ORF-disrupting mutations of Tas1r2, and they occurred independently among the species.[33] They also showed high variance in their lineages.[33] It is hypothesized that the pseudogenization of Tas1r2 occurred through convergent evolution where carnivorous species lost their ability to taste sweet because of dietary behavior.[33]
Umami is also a taste receptor where the function has been lost in many species. The predominant umami taste receptors are Tas1r1/Tas1r3.[33] In two lineages of aquatic mammals including dolphins and sea lions, Tas1r1 has been found to be pseudogenized.[33] The pseudogenization of Tas1r1 has also been found in terrestrial, carnivorous species.[33] While the panda belongs to the order Carnivora, it is herbivorous where 99% of its diet is bamboo, and it cannot taste umami.[35] Genome sequence of the panda shows that its Tas1r1 gene is pseudogenized.[35] In a study, it was found that in all species in the order Carnivora except the panda, the open reading frame was maintained.[35] In panda, the nonsynonymous to synonymous substitutions ratio was found to be much higher than other species in order Carnivora.[35] This data correlates with fossil records date of the panda to show where panda switched from carnivore to herbivore diet.[33] Therefore, the loss of function of umami in panda is hypothesized to be caused by dietary change where the panda became less dependence on meat.[33] However, these studies do not explain herbivores such as horses and cows that have retained the Tas1r1 receptor.[35]
Overall, the loss of function of the a taste receptor is an evolutionary process that occurred due to a dietary change in species.[34]
References
- This, Hervé (2012). "The Science of the Oven - Excerpt from Chapter 1". Retrieved 30 Apr 2014.
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS (August 2001). "Mammalian sweet taste receptors". Cell. 106 (3): 381–90. doi:10.1016/S0092-8674(01)00451-2. PMID 11509186.
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS (March 2000). "A novel family of mammalian taste receptors". Cell. 100 (6): 693–702. doi:10.1016/S0092-8674(00)80705-9. PMID 10761934.
- http://bitterdb.agri.huji.ac.il/dbbitter.php#receptorBrowse
- Steensels S, Depoortere I (2018). "Chemoreceptors in the Gut". Annual Review of Physiology. 80: 117–141. doi:10.1146/annurev-physiol-021317-121332. PMID 29029594.
- Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB (November 2010). "Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction". Nature Medicine. 16 (11): 1299–304. doi:10.1038/nm.2237. PMC 3066567. PMID 20972434.
- Bachmanov AA, Beauchamp GK (2007). "Taste receptor genes". Annual Review of Nutrition. 27: 389–414. doi:10.1146/annurev.nutr.26.061505.111329. PMC 2721271. PMID 17444812.
- Sainz E, Cavenagh MM, LopezJimenez ND, Gutierrez JC, Battey JF, Northup JK, Sullivan SL (June 2007). "The G-protein coupling properties of the human sweet and amino acid taste receptors". Developmental Neurobiology. 67 (7): 948–59. doi:10.1002/dneu.20403. PMID 17506496.
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ (February 2003). "Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways". Cell. 112 (3): 293–301. doi:10.1016/S0092-8674(03)00071-0. PMID 12581520.
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS (March 2002). "An amino-acid taste receptor". Nature. 416 (6877): 199–202. Bibcode:2002Natur.416..199N. doi:10.1038/nature726. PMID 11894099.
- Delay ER, Beaver AJ, Wagner KA, Stapleton JR, Harbaugh JO, Catron KD, Roper SD (October 2000). "Taste preference synergy between glutamate receptor agonists and inosine monophosphate in rats". Chemical Senses. 25 (5): 507–15. doi:10.1093/chemse/25.5.507. PMID 11015322.
- Danilova V, Hellekant G (March 2003). "Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice". BMC Neuroscience. 4: 5. doi:10.1186/1471-2202-4-5. PMC 153500. PMID 12617752.
- Brand JG (April 2000). "Receptor and transduction processes for umami taste". The Journal of Nutrition. 130 (4S Suppl): 942S–5S. doi:10.1093/jn/130.4.942S. PMID 10736357.
- Chaudhari N, Landin AM, Roper SD (February 2000). "A metabotropic glutamate receptor variant functions as a taste receptor". Nature Neuroscience. 3 (2): 113–9. doi:10.1038/72053. PMID 10649565.
- Toyono T, Seta Y, Kataoka S, Kawano S, Shigemoto R, Toyoshima K (July 2003). "Expression of metabotropic glutamate receptor group I in rat gustatory papillae". Cell and Tissue Research. 313 (1): 29–35. doi:10.1007/s00441-003-0740-2. PMID 12898387.
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E (April 2002). "Human receptors for sweet and umami taste". Proceedings of the National Academy of Sciences of the United States of America. 99 (7): 4692–6. Bibcode:2002PNAS...99.4692L. doi:10.1073/pnas.072090199. PMC 123709. PMID 11917125.
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS (October 2003). "The receptors for mammalian sweet and umami taste". Cell. 115 (3): 255–66. doi:10.1016/S0092-8674(03)00844-4. PMID 14636554.
- Hwang LD, Lin C, Gharahkhani P, Cuellar-Partida G, Ong JS, An J, Gordon SD, Zhu G, MacGregor S, Lawlor DA, Breslin PA, Wright MJ, Martin NG, Reed DR (April 2019). "New insight into human sweet taste: a genome-wide association study of the perception and intake of sweet substances". American Journal of Clinical Nutrition. 109: 1724–1737. doi:10.1093/ajcn/nqz043. PMC 6537940. PMID 31005972.
- Yousif, Ragheed (March 2020). "Exploring the Molecular Interactions between Neoculin and the Human Sweet Taste Receptors through Computational Approaches" (PDF). Sains Malaysiana. 49: 517. doi:10.17576/jsm-2020-4903-06.
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ (March 2000). "T2Rs function as bitter taste receptors". Cell. 100 (6): 703–11. doi:10.1016/S0092-8674(00)80706-0. PMID 10761935.
- Margolskee RF (January 2002). "Molecular mechanisms of bitter and sweet taste transduction". The Journal of Biological Chemistry. 277 (1): 1–4. doi:10.1074/jbc.R100054200. PMID 11696554.
- Hwang LD, Gharahkhani P, Breslin PA, Gordon SD, Zhu G, Martin NG, Reed DR, Wright MJ (September 2018). "Bivariate genome-wide association analysis strengthens the role of bitter receptor clusters on chromosomes 7 and 12 in human bitter taste". BMC Genomics. 19 (1): 678. doi:10.1186/s12864-018-5058-2. PMC 6142396. PMID 30223776.
- "Archived copy". Archived from the original on 2015-07-03. Retrieved 2014-10-06.CS1 maint: archived copy as title (link)
- Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P (November 2005). "CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions". The Journal of Clinical Investigation. 115 (11): 3177–84. doi:10.1172/JCI25299. PMC 1265871. PMID 16276419.
- Simons PJ, Kummer JA, Luiken JJ, Boon L (December 2011). "Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae". Acta Histochemica. 113 (8): 839–43. doi:10.1016/j.acthis.2010.08.006. PMID 20950842.
- Baillie AG, Coburn CT, Abumrad NA (September 1996). "Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog". The Journal of Membrane Biology. 153 (1): 75–81. doi:10.1007/s002329900111. PMID 8694909.
- Pepino MY, Love-Gregory L, Klein S, Abumrad NA (March 2012). "The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects". Journal of Lipid Research. 53 (3): 561–6. doi:10.1194/jlr.M021873. PMC 3276480. PMID 22210925.
- DiPatrizio NV (September 2014). "Is fat taste ready for primetime?". Physiology & Behavior. 136: 145–54. doi:10.1016/j.physbeh.2014.03.002. PMC 4162865. PMID 24631296.
- Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S (June 2010). "Taste preference for fatty acids is mediated by GPR40 and GPR120" (PDF). The Journal of Neuroscience. 30 (25): 8376–82. doi:10.1523/JNEUROSCI.0496-10.2010. PMC 6634626. PMID 20573884.
- Mattes RD (September 2011). "Accumulating evidence supports a taste component for free fatty acids in humans". Physiology & Behavior. 104 (4): 624–31. doi:10.1016/j.physbeh.2011.05.002. PMC 3139746. PMID 21557960.
- Liu P, Shah BP, Croasdell S, Gilbertson TA (June 2011). "Transient receptor potential channel type M5 is essential for fat taste". The Journal of Neuroscience. 31 (23): 8634–42. doi:10.1523/JNEUROSCI.6273-10.2011. PMC 3125678. PMID 21653867.
- Feng P, Zhao H (June 2013). "Complex evolutionary history of the vertebrate sweet/umami taste receptor genes". Chinese Science Bulletin. 58 (18): 2198–2204. Bibcode:2013ChSBu..58.2198F. doi:10.1007/s11434-013-5811-5.
- Jiang P, Josue J, Li X, Glaser D, Li W, Brand JG, Margolskee RF, Reed DR, Beauchamp GK (March 2012). "Major taste loss in carnivorous mammals". Proceedings of the National Academy of Sciences of the United States of America. 109 (13): 4956–61. doi:10.1073/pnas.1118360109. PMC 3324019. PMID 22411809.
- Antinucci M, Risso D (2017-11-28). "A Matter of Taste: Lineage-Specific Loss of Function of Taste Receptor Genes in Vertebrates". Frontiers in Molecular Biosciences. 4: 81. doi:10.3389/fmolb.2017.00081. PMC 5712339. PMID 29234667.
- Zhao H, Yang JR, Xu H, Zhang J (December 2010). "Pseudogenization of the umami taste receptor gene Tas1r1 in the giant panda coincided with its dietary switch to bamboo". Molecular Biology and Evolution. 27 (12): 2669–73. doi:10.1093/molbev/msq153. PMC 3108379. PMID 20573776.
External links
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba JP, Zucker CS, Patton A (2000). "A Novel Family of Mammalian Taste Receptors - An Investigative Review". Davidson College Biology Department. Retrieved 2008-08-11.
- taste+receptors,+type+1 at the US National Library of Medicine Medical Subject Headings (MeSH)
- taste+receptors,+type+2 at the US National Library of Medicine Medical Subject Headings (MeSH)