Methoxyketamine
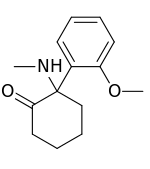
Methoxyketamine or 2-MeO-2-deschloroketamine is a designer drug of the arylcyclohexylamine class first reported in 1963.[1] It is an analog of ketamine in which the chlorine atom has been replaced with a methoxy group. Its synthesis by rearrangement of an amino ketone has been reported.[2] As an arylcyclohexylamine, methoxyketamine most likely functions as an NMDA receptor antagonist. It produces sedative, hallucinogenic, and (at high doses) anesthetic effects, but with a lower potency than ketamine itself.
Not to be confused with Methoxetamine.
 | |
| Names | |
|---|---|
| IUPAC name
2-(2-Methoxyphenyl)-2-(methylamino)cyclohexanone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H19NO2 | |
| Molar mass | 233.311 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- BE 634208, Stevens, Calvin L., "Amino ketones", published 1963
- Stevens, Calvin L.; Thuillier, Andre; Taylor, K. Grant; Daniher, Francis A.; Dickerson, James P.; Hanson, Harry T.; Nielsen, Norman A.; Tikotkar, N. A.; Weier, Richard M. (1966). "Amino Ketone Rearrangements. VII.1 Synthesis of 2-Methylamino-2-Substituted Phenylcyclohexanones". The Journal of Organic Chemistry. 31 (8): 2601. doi:10.1021/jo01346a034.
| |||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.