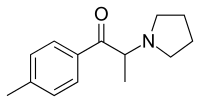
4'-Methyl-α-pyrrolidinopropiophenone
4'-Methyl-α-pyrrolidinopropiophenone (4-MePPP, MPPP or MαPPP) is a stimulant drug and substituted cathinone. It is structurally very similar to α-PPP, with only one added methyl group in the para position on the phenyl ring. 4-MePPP was sold in Germany as a designer drug in the late 1990s and early 2000s,[2][3][4] along with a number of other pyrrolidinophenone derivatives.[5][6] Although it has never achieved the same international popularity as its better-known relations α-PPP and MDPV, 4-MePPP is still sometimes found as an ingredient of grey-market "bath salt" blends[7] such as "NRG-3".[7]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H19NO |
| Molar mass | 217.312 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
- α-Pyrrolidinopropiophenone (α-PPP)
- 4'-Methoxy-α-pyrrolidinopropiophenone (MOPPP)
- 3,4-Methylenedioxy-α-pyrrolidinopropiophenone (MDPPP)
- 3',4'-Methylenedioxy-α-pyrrolidinobutiophenone (MDPBP)
References
- "Cannabinoider föreslås bli klassade som hälsofarlig vara". Retrieved 29 June 2015.
- Springer, D; Peters, FT; Fritschi, G; Maurer, HH (2002). "Studies on the metabolism and toxicological detection of the new designer drug 4'-methyl-alpha-pyrrolidinopropiophenone in urine using gas chromatography-mass spectrometry". Journal of Chromatography B. 773 (1): 25–33. doi:10.1016/S1570-0232(01)00578-5. PMID 12015267.
- Springer, D; Paul, LD; Staack, RF; Kraemer, T; Maurer, HH (2003). "Identification of cytochrome p450 enzymes involved in the metabolism of 4'-methyl-alpha-pyrrolidinopropiophenone, a novel scheduled designer drug, in human liver microsomes". Drug Metabolism and Disposition. 31 (8): 979–82. doi:10.1124/dmd.31.8.979. PMID 12867484.
- Springer, D; Fritschi, G; Maurer, HH (2003). "Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4'-methyl-alpha-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometry". Journal of Chromatography B. 796 (2): 253–66. doi:10.1016/j.jchromb.2003.07.008. PMID 14581066.
- Maurer, HH; Kraemer, T; Springer, D; Staack, RF (2004). "Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: a synopsis". Therapeutic drug monitoring. 26 (2): 127–31. doi:10.1097/00007691-200404000-00007. PMID 15228152.
- Staack, RF; Maurer, HH (2005). "Metabolism of designer drugs of abuse". Current Drug Metabolism. 6 (3): 259–74. doi:10.2174/1389200054021825. PMID 15975043.
- Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J (December 2010). "Analysis of NRG 'legal highs' in the UK: identification and formation of novel cathinones". Drug Testing and Analysis. 3 (9): 569–75. doi:10.1002/dta.204. PMID 21960541.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
