NKX3-1
Homeobox protein Nkx-3.1, also known as NKX3-1, NKX3, BAPX2, NKX3A and NKX3.1 is a protein that in humans is encoded by the NKX3-1 gene located on chromosome 8p.[5] NKX3-1 is a prostatic tumor suppressor gene.
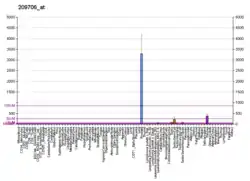
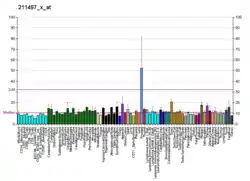
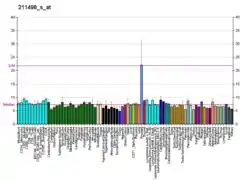
NKX3-1 is an androgen-regulated, prostate-specific homeobox gene whose expression is predominantly localized to prostate epithelium. It acts as a transcription factor that has critical function in prostate development and tumor suppression. It is a negative regulator of epithelial cell growth in prostate tissue. The NKX3-1 homeobox protein is encoded by the NKX3-1 gene.[5]
Function
The homeodomain-containing transcription factor NKX3A is a putative prostate tumor suppressor that is expressed in a largely prostate-specific and androgen-regulated manner. Loss of NKX3A protein expression is a common finding in human prostate carcinomas and prostatic intraepithelial neoplasia.[6]
Gene
In humans, the NKX3-1 gene is located on chromosome 8p21.2 with 4 exons.[7] The 8p chromosome is a region that is frequently reported to undergo a loss of heterozygosity (LOH) associated with tissue dedifferentiation and loss of androgen responsiveness during the progression of prostate cancer. LOH has been reported to be observed in 12-89% of high-grade prostatic intraepithelial neoplasia (PIN) and 35-86% of prostatic adenocarcinomas. The frequency of loss of heterozygosity on chromosome 8p is seen to increase with advanced prostate cancer grade and stage.[8]
Structure
NKX3-1 contains two exons encoding a 234 amino acid protein including a homeodomain. The 234 amino acids are 35-38 kDa. One N-terminal domain one homeodomain and one C-terminal domain are present. The observed interaction between NKX3-1 and Serum Response Factor (SRF)indicate that amino-terminal domains participate in the interaction. The synergistic transcriptional activation requires both interactions at multiple protein-protein interfaces and protein-DNA interactions. This indicates that one mechanism of NKX3-1 dependent transcriptional activation in prostate epithelia requires combinatorial interactions with other factors expressed within those cells[9]
In 2000, full length NKX3-1 cDNA was obtained from a human prostate cDNA library. Korkmaz et al.[10] identified 3 splice variants with deletions in the N-terminal region as well as a variant at position 137 within the homeobox domain. NKX3-1 expression was visualized using Fluorescence microscopy, utilizing GFP-NKX3-1 in the nucleus.
Function
NKX3-1 expression acts as a transcription factor that has been found to play a main role in prostate development and tumor suppression. The loss of NKX3-1 expression is frequently observed in prostate tumorigenesis and has been seen to be a result of allelic loss, methylation, and post transcriptional silencing.[11] NKX3-1 expression is seen in prostate epithelium, testis, ureter, and pulmonary bronchial mucous glands.
NKX3-1 binds to DNA to suppress transcription as well as interacts with transcription factors such as serum response factor, to enhance transcriptional activation. Wang et al.[12] demonstrated that NKX3-1 marks a stem cell population that functions during prostate regeneration. Genetic lineage marking demonstrated that rare luminal cells that express NKX3-1 in the absence of testicular androgens are bipotential and can self-renew in vivo. Single-cell transplantation assays showed that castration-resistant NKX3-1 expressing cells (CARNs) can reconstitute prostate ducts in renal grafts. Functional assays of NKX3-1 mutant mice in serial prostate regeneration suggested that NKX3-1 is required for stem cell maintenance. Furthermore, targeted deletion of PTEN gene in CARNs resulted in rapid carcinoma formation after androgen-mediated regeneration. This indicates that CARNs represent a new luminal stem cell population that is an efficient target for oncogenic transformation in prostate cancer.
It has also been found to be essential in pluripotency of stem cells using Yamanaka factors.[13]
Regulation
In 2010 it was shown that NKX3-1 was controlled by ERG and ESE3 both directly and through induction of EZH2 (Polycomb group pcg).[14]
Discovery
Using a random cDNA sequencing approach, He et al.[15] cloned a novel prostate-specific gene that encoded a homeobox-containing protein. The gene which they symbolized NKX3-1 encoded a 234-amino acid polypeptide with greatest homology to the Drosophila NK3 gene. Northern blot analysis showed that NKX3.1 had a uniquely restricted tissue expression pattern with mRNA being abundant in the prostate, lower levels in the testis and absent from all other tissues tested. The NKX3-1 protein expression was detected a hormone-responsive, androgen receptor-positive prostate cancer cell line, but was absent from androgen receptor-negative prostate cancer cell lines as well as other cell lines of varied origins. The link between androgen stimulation and NKX3-1 was discovered through the use of an androgen-dependent carcinoma line. The researchers suggested that the NKX3-1 gene plays a role in androgen-driven differentiation of prostatic tissue as well as in loss of differentiation during the progression of prostate cancer.
Role in disease
Prostate cancer is the most commonly diagnosed cancer in American men and the second leading cause of cancer related deaths.[16] Prostate cancer predominantly occurs in the peripheral zone of the human prostate, with fewer than 10% of cases found in the central zone. The disease develops as a result of the temporal and spatial loss of the basal epithelial compartment as well as increased proliferation and dedifferentiation of the luminal (secretory) epithelial cells. Prostate cancer is typically found in men of ages older than 60 and its incidence increases with increasing age.
NKX3-1 plays an essential role in normal murine prostate development. Loss of function of NKX3-1 leads to defects in prostatic protein secretions as well as ductal morphogenesis. Loss of function also contributes to prostate carcinogenesis.
NKX3-1 has been established as a marker for identifying metastatic tumors.[8] Furthermore, anti-NKX3-1 antibodies are a more sensitive and specific method for diagnosing metastatic prostatic adenocarcinomas in distant sites.[17]
Interactions
NKX3-1 has been shown to interact with SPDEF.[18]
The stability of NKX3-1 protein has been shown to be regulated by phosphorylation.[19]
References
- GRCh38: Ensembl release 89: ENSG00000167034 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000022061 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- He WW, Sciavolino PJ, Wing J, Augustus M, Hudson P, Meissner PS, Curtis RT, Shell BK, Bostwick DG, Tindall DJ, Gelmann EP, Abate-Shen C, Carter KC (Jul 1997). "A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer". Genomics. 43 (1): 69–77. doi:10.1006/geno.1997.4715. PMID 9226374.
- "Entrez Gene: NKX3-1 NK3 transcription factor related, locus 1 (Drosophila)".
- "NCBI - WWW Error Blocked Diagnostic". www.ncbi.nlm.nih.gov.
- Gurel B, Ali TZ, Montgomery EA, Begum S, Hicks J, Goggins M, Eberhart CG, Clark DP, Bieberich CJ, Epstein JI, De Marzo AM (Aug 2010). "NKX3.1 as a marker of prostatic origin in metastatic tumors". The American Journal of Surgical Pathology. 34 (8): 1097–1105. doi:10.1097/PAS.0b013e3181e6cbf3. PMC 3072223. PMID 20588175.
- Zhang Y, Fillmore RA, Zimmer WE (Mar 2008). "Structural and functional analysis of domains mediating interaction between the bagpipe homologue, Nkx3.1 and serum response factor". Experimental Biology and Medicine. 233 (3): 297–309. doi:10.3181/0709-RM-236. PMID 18296735. S2CID 9377426.
- Korkmaz KS, Korkmaz CG, Ragnhildstveit E, Kizildag S, Pretlow TG, Saatcioglu F (Dec 2000). "Full-length cDNA sequence and genomic organization of human NKX3A - alternative forms and regulation by both androgens and estrogens". Gene. 260 (1–2): 25–36. doi:10.1016/S0378-1119(00)00453-4. PMID 11137288.
- Abate-Shen C, Shen MM, Gelmann E (Jul 2008). "Integrating differentiation and cancer: the Nkx3.1 homeobox gene in prostate organogenesis and carcinogenesis". Differentiation; Research in Biological Diversity. 76 (6): 717–727. doi:10.1111/j.1432-0436.2008.00292.x. PMC 3683569. PMID 18557759.
- Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM (Sep 2009). "A luminal epithelial stem cell that is a cell of origin for prostate cancer". Nature. 461 (7263): 495–500. Bibcode:2009Natur.461..495W. doi:10.1038/nature08361. PMC 2800362. PMID 19741607.
- "Researchers identify protein essential for making stem cells".
- Kunderfranco P, Mello-Grand M, Cangemi R, Pellini S, Mensah A, Albertini V, Malek A, Chiorino G, Catapano CV, Carbone GM (2010). "ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancer". PLOS ONE. 5 (5): e10547. Bibcode:2010PLoSO...510547K. doi:10.1371/journal.pone.0010547. PMC 2866657. PMID 20479932.
- He WW, Sciavolino PJ, Wing J, Augustus M, Hudson P, Meissner PS, Curtis RT, Shell BK, Bostwick DG, Tindall DJ, Gelmann EP, Abate-Shen C, Carter KC (Jul 1997). "A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer". Genomics. 43 (1): 69–77. doi:10.1006/geno.1997.4715. PMID 9226374.
- "http://www.cancer.org/cancer/prostatecancer/". www.cancer.org. External link in
|title=(help) - Chuang AY, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI (Aug 2007). "Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma". The American Journal of Surgical Pathology. 31 (8): 1246–1255. doi:10.1097/PAS.0b013e31802f5d33. PMID 17667550. S2CID 11535862.
- Chen H, Nandi AK, Li X, Bieberich CJ (Jan 2002). "NKX-3.1 interacts with prostate-derived Ets factor and regulates the activity of the PSA promoter". Cancer Research. 62 (2): 338–40. PMID 11809674.
- Padmanabhan A, Gosc EB, Bieberich CJ (May 2013). "Stabilization of the prostate-specific tumor suppressor NKX3.1 by the oncogenic protein kinase Pim-1 in prostate cancer cells". Journal of Cellular Biochemistry. 114 (5): 1050–7. doi:10.1002/jcb.24444. PMID 23129228. S2CID 29814674.
Further reading
- Shen MM, Abate-Shen C (Dec 2003). "Roles of the Nkx3.1 homeobox gene in prostate organogenesis and carcinogenesis". Developmental Dynamics. 228 (4): 767–78. doi:10.1002/dvdy.10397. PMID 14648854. S2CID 6303940.
- Abdulkadir SA (Nov 2005). "Mechanisms of prostate tumorigenesis: roles for transcription factors Nkx3.1 and Egr1". Annals of the New York Academy of Sciences. 1059 (1): 33–40. Bibcode:2005NYASA1059...33A. doi:10.1196/annals.1339.018. PMID 16382041. S2CID 6774788.
- Voeller HJ, Augustus M, Madike V, Bova GS, Carter KC, Gelmann EP (Oct 1997). "Coding region of NKX3.1, a prostate-specific homeobox gene on 8p21, is not mutated in human prostate cancers". Cancer Research. 57 (20): 4455–9. PMID 9377551.
- Prescott JL, Blok L, Tindall DJ (Apr 1998). "Isolation and androgen regulation of the human homeobox cDNA, NKX3.1". The Prostate. 35 (1): 71–80. doi:10.1002/(SICI)1097-0045(19980401)35:1<71::AID-PROS10>3.0.CO;2-H. PMID 9537602.
- Choi CY, Kim YH, Kwon HJ, Kim Y (Nov 1999). "The homeodomain protein NK-3 recruits Groucho and a histone deacetylase complex to repress transcription". The Journal of Biological Chemistry. 274 (47): 33194–7. doi:10.1074/jbc.274.47.33194. PMID 10559189.
- Carson JA, Fillmore RA, Schwartz RJ, Zimmer WE (Dec 2000). "The smooth muscle gamma-actin gene promoter is a molecular target for the mouse bagpipe homologue, mNkx3-1, and serum response factor". The Journal of Biological Chemistry. 275 (50): 39061–72. doi:10.1074/jbc.M006532200. PMID 10993896.
- Bowen C, Bubendorf L, Voeller HJ, Slack R, Willi N, Sauter G, Gasser TC, Koivisto P, Lack EE, Kononen J, Kallioniemi OP, Gelmann EP (Nov 2000). "Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression". Cancer Research. 60 (21): 6111–5. PMID 11085535.
- Korkmaz KS, Korkmaz CG, Ragnhildstveit E, Kizildag S, Pretlow TG, Saatcioglu F (Dec 2000). "Full-length cDNA sequence and genomic organization of human NKX3A - alternative forms and regulation by both androgens and estrogens". Gene. 260 (1–2): 25–36. doi:10.1016/S0378-1119(00)00453-4. PMID 11137288.
- Chen H, Nandi AK, Li X, Bieberich CJ (Jan 2002). "NKX-3.1 interacts with prostate-derived Ets factor and regulates the activity of the PSA promoter". Cancer Research. 62 (2): 338–40. PMID 11809674.
- Filmore RA, Dean DA, Zimmer WE (2003). "The smooth muscle gamma-actin gene is androgen responsive in prostate epithelia". Gene Expression. 10 (5–6): 201–11. doi:10.3727/000000002783992424. PMC 5977519. PMID 12450213.
- Gelmann EP, Bowen C, Bubendorf L (May 2003). "Expression of NKX3.1 in normal and malignant tissues". The Prostate. 55 (2): 111–7. doi:10.1002/pros.10210. PMID 12661036. S2CID 24920337.
- Skotheim RI, Korkmaz KS, Klokk TI, Abeler VM, Korkmaz CG, Nesland JM, Fosså SD, Lothe RA, Saatcioglu F (Dec 2003). "NKX3.1 expression is lost in testicular germ cell tumors". The American Journal of Pathology. 163 (6): 2149–54. doi:10.1016/S0002-9440(10)63571-7. PMC 1892359. PMID 14633588.
- Korkmaz CG, Korkmaz KS, Manola J, Xi Z, Risberg B, Danielsen H, Kung J, Sellers WR, Loda M, Saatcioglu F (Sep 2004). "Analysis of androgen regulated homeobox gene NKX3.1 during prostate carcinogenesis". The Journal of Urology. 172 (3): 1134–9. doi:10.1097/01.ju.0000136526.78535.b8. PMID 15311057.
- Chen H, Bieberich CJ (Jan 2005). "Structural and functional analysis of domains mediating interaction between NKX-3.1 and PDEF". Journal of Cellular Biochemistry. 94 (1): 168–77. doi:10.1002/jcb.20297. PMID 15523673. S2CID 46494570.
- Lind GE, Skotheim RI, Fraga MF, Abeler VM, Henrique R, Saatcioglu F, Esteller M, Teixeira MR, Lothe RA (Feb 2005). "The loss of NKX3.1 expression in testicular--and prostate--cancers is not caused by promoter hypermethylation". Molecular Cancer. 4 (1): 8. doi:10.1186/1476-4598-4-8. PMC 548671. PMID 15691383.
- Asatiani E, Huang WX, Wang A, Rodriguez Ortner E, Cavalli LR, Haddad BR, Gelmann EP (Feb 2005). "Deletion, methylation, and expression of the NKX3.1 suppressor gene in primary human prostate cancer". Cancer Research. 65 (4): 1164–73. doi:10.1158/0008-5472.CAN-04-2688. PMID 15734999.
External links
- NKX3-1+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.