E2F1
Transcription factor E2F1 is a protein that in humans is encoded by the E2F1 gene.[5]
Function
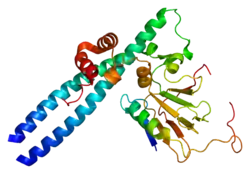
The protein encoded by this gene is a member of the E2F family of transcription factors. The E2F family plays a crucial role in the control of cell cycle and action of tumor suppressor proteins and is also a target of the transforming proteins of small DNA tumor viruses. The E2F proteins contain several evolutionarily conserved domains found in most members of the family. These domains include a DNA binding domain, a dimerization domain which determines interaction with the differentiation regulated transcription factor proteins (DP), a transactivation domain enriched in acidic amino acids, and a tumor suppressor protein association domain which is embedded within the transactivation domain. This protein and another 2 members, E2F2 and E2F3, have an additional cyclin binding domain. This protein binds preferentially to retinoblastoma protein pRB in a cell-cycle dependent manner. It can mediate both cell proliferation and p53-dependent/independent apoptosis.[6]
Interactions
E2F1 has been shown to interact with:
See also
References
- GRCh38: Ensembl release 89: ENSG00000101412 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000027490 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Neuman E, Sellers WR, McNeil JA, Lawrence JB, Kaelin WG (December 1996). "Structure and partial genomic sequence of the human E2F1 gene". Gene. 173 (2): 163–9. doi:10.1016/0378-1119(96)00184-9. PMID 8964493.
- "Entrez Gene: E2F1 E2F transcription factor 1".
- Suzuki M, Okuyama S, Okamoto S, Shirasuna K, Nakajima T, Hachiya T, Nojima H, Sekiya S, Oda K (August 1998). "A novel E2F binding protein with Myc-type HLH motif stimulates E2F-dependent transcription by forming a heterodimer". Oncogene. 17 (7): 853–65. doi:10.1038/sj.onc.1202163. PMID 9780002.
- Marti A, Wirbelauer C, Scheffner M, Krek W (May 1999). "Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation". Nat. Cell Biol. 1 (1): 14–9. doi:10.1038/8984. PMID 10559858. S2CID 8884226.
- Yang R, Müller C, Huynh V, Fung YK, Yee AS, Koeffler HP (March 1999). "Functions of cyclin A1 in the cell cycle and its interactions with transcription factor E2F-1 and the Rb family of proteins". Mol. Cell. Biol. 19 (3): 2400–7. doi:10.1128/mcb.19.3.2400. PMC 84032. PMID 10022926.
- Xu M, Sheppard KA, Peng CY, Yee AS, Piwnica-Worms H (December 1994). "Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation". Mol. Cell. Biol. 14 (12): 8420–31. doi:10.1128/MCB.14.12.8420. PMC 359381. PMID 7969176.
- Vandel L, Kouzarides T (August 1999). "Residues phosphorylated by TFIIH are required for E2F-1 degradation during S-phase". EMBO J. 18 (15): 4280–91. doi:10.1093/emboj/18.15.4280. PMC 1171504. PMID 10428966.
- Strachan GD, Jordan-Sciutto KL, Rallapalli R, Tuan RS, Hall DJ (February 2003). "The E2F-1 transcription factor is negatively regulated by its interaction with the MDMX protein". J. Cell. Biochem. 88 (3): 557–68. doi:10.1002/jcb.10318. PMID 12532331. S2CID 38805122.
- Kong HJ, Yu HJ, Hong S, Park MJ, Choi YH, An WG, Lee JW, Cheong J (November 2003). "Interaction and functional cooperation of the cancer-amplified transcriptional coactivator activating signal cointegrator-2 and E2F-1 in cell proliferation". Mol. Cancer Res. 1 (13): 948–58. PMID 14638867.
- Taniura H, Taniguchi N, Hara M, Yoshikawa K (January 1998). "Necdin, a postmitotic neuron-specific growth suppressor, interacts with viral transforming proteins and cellular transcription factor E2F1". J. Biol. Chem. 273 (2): 720–8. doi:10.1074/jbc.273.2.720. PMID 9422723.
- Kuwako K, Taniura H, Yoshikawa K (January 2004). "Necdin-related MAGE proteins differentially interact with the E2F1 transcription factor and the p75 neurotrophin receptor". J. Biol. Chem. 279 (3): 1703–12. doi:10.1074/jbc.M308454200. PMID 14593116.
- Sansal I, Dupont E, Toru D, Evrard C, Rouget P (October 2000). "NPDC-1, a regulator of neural cell proliferation and differentiation, interacts with E2F-1, reduces its binding to DNA and modulates its transcriptional activity". Oncogene. 19 (43): 5000–9. doi:10.1038/sj.onc.1203843. PMID 11042687.
- Darbinian N, Gallia GL, Kundu M, Shcherbik N, Tretiakova A, Giordano A, Khalili K (November 1999). "Association of Pur alpha and E2F-1 suppresses transcriptional activity of E2F-1". Oncogene. 18 (46): 6398–402. doi:10.1038/sj.onc.1203011. PMID 10597240.
- Joshi B, Ko D, Ordonez-Ercan D, Chellappan SP (December 2003). "A putative coiled-coil domain of prohibitin is sufficient to repress E2F1-mediated transcription and induce apoptosis". Biochem. Biophys. Res. Commun. 312 (2): 459–66. doi:10.1016/j.bbrc.2003.10.148. PMID 14637159.
- Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S (November 2003). "Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling". J. Biol. Chem. 278 (48): 47853–61. doi:10.1074/jbc.M305171200. PMID 14500729.
- Wang S, Zhang B, Faller DV (June 2002). "Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth". EMBO J. 21 (12): 3019–28. doi:10.1093/emboj/cdf302. PMC 126057. PMID 12065415.
- Wang S, Nath N, Fusaro G, Chellappan S (November 1999). "Rb and prohibitin target distinct regions of E2F1 for repression and respond to different upstream signals". Mol. Cell. Biol. 19 (11): 7447–60. doi:10.1128/mcb.19.11.7447. PMC 84738. PMID 10523633.
- Dyson N, Dembski M, Fattaey A, Ngwu C, Ewen M, Helin K (December 1993). "Analysis of p107-associated proteins: p107 associates with a form of E2F that differs from pRB-associated E2F-1". J. Virol. 67 (12): 7641–7. doi:10.1128/JVI.67.12.7641-7647.1993. PMC 238233. PMID 8230483.
- Nicolas E, Ait-Si-Ali S, Trouche D (August 2001). "The histone deacetylase HDAC3 targets RbAp48 to the retinoblastoma protein". Nucleic Acids Res. 29 (15): 3131–6. doi:10.1093/nar/29.15.3131. PMC 55834. PMID 11470869.
- Pardo PS, Leung JK, Lucchesi JC, Pereira-Smith OM (December 2002). "MRG15, a novel chromodomain protein, is present in two distinct multiprotein complexes involved in transcriptional activation". J. Biol. Chem. 277 (52): 50860–6. doi:10.1074/jbc.M203839200. PMID 12397079.
- Choubey D, Li SJ, Datta B, Gutterman JU, Lengyel P (October 1996). "Inhibition of E2F-mediated transcription by p202". EMBO J. 15 (20): 5668–78. doi:10.1002/j.1460-2075.1996.tb00951.x. PMC 452311. PMID 8896460.
- Fajas L, Paul C, Zugasti O, Le Cam L, Polanowska J, Fabbrizio E, Medema R, Vignais ML, Sardet C (July 2000). "pRB binds to and modulates the transrepressing activity of the E1A-regulated transcription factor p120E4F". Proc. Natl. Acad. Sci. U.S.A. 97 (14): 7738–43. Bibcode:2000PNAS...97.7738F. doi:10.1073/pnas.130198397. PMC 16614. PMID 10869426.
- Wu CL, Zukerberg LR, Ngwu C, Harlow E, Lees JA (May 1995). "In vivo association of E2F and DP family proteins". Mol. Cell. Biol. 15 (5): 2536–46. doi:10.1128/mcb.15.5.2536. PMC 230484. PMID 7739537.
- Rotheneder H, Geymayer S, Haidweger E (November 1999). "Transcription factors of the Sp1 family: interaction with E2F and regulation of the murine thymidine kinase promoter". J. Mol. Biol. 293 (5): 1005–15. doi:10.1006/jmbi.1999.3213. PMID 10547281.
- Lin SY, Black AR, Kostic D, Pajovic S, Hoover CN, Azizkhan JC (April 1996). "Cell cycle-regulated association of E2F1 and Sp1 is related to their functional interaction". Mol. Cell. Biol. 16 (4): 1668–75. doi:10.1128/MCB.16.4.1668. PMC 231153. PMID 8657142.
- Karlseder J, Rotheneder H, Wintersberger E (April 1996). "Interaction of Sp1 with the growth- and cell cycle-regulated transcription factor E2F". Mol. Cell. Biol. 16 (4): 1659–67. doi:10.1128/mcb.16.4.1659. PMC 231152. PMID 8657141.
- Sardet C, Vidal M, Cobrinik D, Geng Y, Onufryk C, Chen A, Weinberg RA (March 1995). "E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle". Proc. Natl. Acad. Sci. U.S.A. 92 (6): 2403–7. Bibcode:1995PNAS...92.2403S. doi:10.1073/pnas.92.6.2403. PMC 42492. PMID 7892279.
- Helin K, Wu CL, Fattaey AR, Lees JA, Dynlacht BD, Ngwu C, Harlow E (October 1993). "Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative trans-activation". Genes Dev. 7 (10): 1850–61. doi:10.1101/gad.7.10.1850. PMID 8405995.
- Liu K, Lin FT, Ruppert JM, Lin WC (May 2003). "Regulation of E2F1 by BRCT domain-containing protein TopBP1". Mol. Cell. Biol. 23 (9): 3287–304. doi:10.1128/mcb.23.9.3287-3304.2003. PMC 153207. PMID 12697828.
- Yu X, Chini CC, He M, Mer G, Chen J (October 2003). "The BRCT domain is a phospho-protein binding domain". Science. 302 (5645): 639–42. Bibcode:2003Sci...302..639Y. doi:10.1126/science.1088753. PMID 14576433. S2CID 29407635.
- Zhou F, Zhang L, Wang A, Song B, Gong K, Zhang L, Hu M, Zhang X, Zhao N, Gong Y (May 2008). "The association of GSK3 beta with E2F1 facilitates nerve growth factor-induced neural cell differentiation". J. Biol. Chem. 283 (21): 14506–15. doi:10.1074/jbc.M706136200. PMID 18367454.
Further reading
- Dupont E, Sansal I, Toru D, Evrard C, Rouget P (1997). "[Identification of NPDC-1, gene involved in the control of proliferation and differentiation of neural and glial precursors]". C. R. Séances Soc. Biol. Fil. 191 (1): 95–104. PMID 9181131.
- Stevens C, La Thangue NB (2005). "The emerging role of E2F-1 in the DNA damage response and checkpoint control". DNA Repair (Amst.). 3 (8–9): 1071–9. doi:10.1016/j.dnarep.2004.03.034. PMID 15279795.
- Zhang Z, Wang H, Li M, Rayburn E, Agrawal S, Zhang R (2006). "Novel MDM2 p53-independent functions identified through RNA silencing technologies". Ann. N. Y. Acad. Sci. 1058 (1): 205–14. doi:10.1196/annals.1359.030. PMID 16394138. S2CID 35683657.
- Schild C, Wirth M, Reichert M, Schmid RM, Saur D, Schneider G (July 2009). "PI3K signaling maintains c-myc expression to regulate transcription of E2F1 in pancreatic cancer cells". Mol. Carcinog. 48 (12): 1149–58. doi:10.1002/mc.20569. PMID 19603422. S2CID 41545085.
External links
- E2F1+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
- FactorBook E2F1
This article incorporates text from the United States National Library of Medicine, which is in the public domain.