ASCL1
Achaete-scute homolog 1 is a protein that in humans is encoded by the ASCL1 gene.[5][6] Because it was discovered subsequent to studies on its homolog in Drosophila, the Achaete-scute complex, it was originally named MASH-1 for mammalian achaete scute homolog-1.[7]
Function
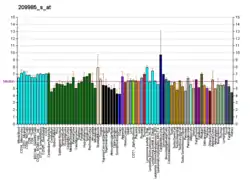
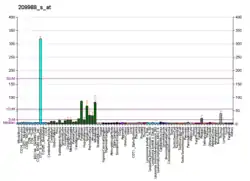
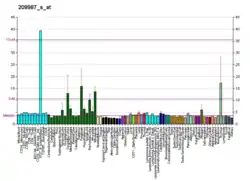
This gene encodes a member of the basic helix-loop-helix (BHLH) family of transcription factors. The protein activates transcription by binding to the E box (5'-CANNTG-3'). Dimerization with other BHLH proteins is required for efficient DNA binding. This protein plays a role in the neuronal commitment and differentiation and in the generation of olfactory and autonomic neurons. It is highly expressed in medullary thyroid cancer and small cell lung cancer and may be a useful marker for these cancers. The presence of a CAG repeat in the gene suggests that it may also play a role in tumor formation.[6]
Role in neuronal commitment
Development of the vertebrate nervous system begins when the neural tube forms in the early embryo. The neural tube eventually gives rise to the entire nervous system, but first neuroblasts must differentiate from the neuroepithelium of the tube. The neuroblasts are the cells that undergo mitotic division and produce neurons.[7] Asc is central to the differentiation of the neuroblasts and the lateral inhibition mechanism which inherently creates a safety net in the event of damage or death in these incredibly important cells.[7]
Differentiation of the neuroblast begins when the cells of the neural tube express Asc and thus upregulate the expression of Delta, a protein essential to the lateral inhibition pathway of neuronal commitment.[7] Delta can diffuse to neighboring cells and bind to the Notch receptor, a large transmembrane protein which upon activation undergoes proteolytic cleavage to release the intracellular domain (Notch-ICD).[7] The Notch-ICD is then free to travel to the nucleus and form a complex with Suppressor of Hairless (SuH) and Mastermind.[7] This complex acts as transcription regulator of Asc and accomplishes two important tasks. First, it prevents the expression of factors required for differentiation of the cell into a neuroblast.[7] Secondly, it inhibits the neighboring cell's production of Delta.[7] Therefore, the future neuroblast will be the cell that has the greatest Asc activation in the vicinity and consequently the greatest Delta production that will inhibit the differentiation of neighboring cells. The select group of neuroblasts that then differentiate in the neural tube are thus replaceable because the neuroblast's ability to suppress differentiation of neighboring cells depends on its own ability to produce Asc.[7] This process of neuroblast differentiation via Asc is common to all animals.[7] Although this mechanism was initially studied in Drosophila, homologs to all proteins in the pathway have been found in vertebrates that have the same bHLH structure.[7]
Autonomic nervous system development
In addition to its important role in neuroblast formation, Asc also functions to mediate autonomic nervous system (ANS) formation.[8] Asc was initially suspected to play a role in the ANS when ASCL1 was found expressed in cells surrounding the dorsal aorta, the adrenal glands and in the developing sympathetic chain during a specific stage of development.[8] Subsequent studies of mice genetically altered to be MASH-1 deficient revealed defective development of both sympathetic and parasympathetic ganglia, the two constituents of the ANS.[8]
Interactions
ASCL1 has been shown to interact with Myocyte-specific enhancer factor 2A.[9]
References
- GRCh38: Ensembl release 89: ENSG00000139352 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000020052 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Ball DW, Azzoli CG, Baylin SB, Chi D, Dou S, Donis-Keller H, et al. (June 1993). "Identification of a human achaete-scute homolog highly expressed in neuroendocrine tumors". Proceedings of the National Academy of Sciences of the United States of America. 90 (12): 5648–52. Bibcode:1993PNAS...90.5648B. doi:10.1073/pnas.90.12.5648. PMC 46778. PMID 8390674.
- "Entrez Gene: ASCL1 achaete-scute complex homolog 1 (Drosophila)".
- Sanes DH (2011). The development of the nervous system. Elsevier. ISBN 978-0-12-374539-2.
- Axelson H (February 2004). "The Notch signaling cascade in neuroblastoma: role of the basic helix-loop-helix proteins HASH-1 and HES-1". Cancer Letters. 204 (2): 171–8. doi:10.1016/s0304-3835(03)00453-1. PMID 15013216.
- Mao Z, Nadal-Ginard B (June 1996). "Functional and physical interactions between mammalian achaete-scute homolog 1 and myocyte enhancer factor 2A". The Journal of Biological Chemistry. 271 (24): 14371–5. doi:10.1074/jbc.271.24.14371. PMID 8662987.
Further reading
- Chen H, Kunnimalaiyaan M, Van Gompel JJ (June 2005). "Medullary thyroid cancer: the functions of raf-1 and human achaete-scute homologue-1". Thyroid. 15 (6): 511–21. doi:10.1089/thy.2005.15.511. PMID 16029117.
- Renault B, Lieman J, Ward D, Krauter K, Kucherlapati R (November 1995). "Localization of the human achaete-scute homolog gene (ASCL1) distal to phenylalanine hydroxylase (PAH) and proximal to tumor rejection antigen (TRA1) on chromosome 12q22-q23". Genomics. 30 (1): 81–3. doi:10.1006/geno.1995.0012. PMID 8595908.
- Mao Z, Nadal-Ginard B (June 1996). "Functional and physical interactions between mammalian achaete-scute homolog 1 and myocyte enhancer factor 2A". The Journal of Biological Chemistry. 271 (24): 14371–5. doi:10.1074/jbc.271.24.14371. PMID 8662987.
- Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, et al. (April 1997). "An achaete-scute homologue essential for neuroendocrine differentiation in the lung". Nature. 386 (6627): 852–5. Bibcode:1997Natur.386..852B. doi:10.1038/386852a0. PMID 9126746. S2CID 4336900.
- Chen H, Biel MA, Borges MW, Thiagalingam A, Nelkin BD, Baylin SB, Ball DW (June 1997). "Tissue-specific expression of human achaete-scute homologue-1 in neuroendocrine tumors: transcriptional regulation by dual inhibitory regions". Cell Growth & Differentiation. 8 (6): 677–86. PMID 9186001.
- Lo L, Sommer L, Anderson DJ (June 1997). "MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells". Current Biology. 7 (6): 440–50. doi:10.1016/S0960-9822(06)00191-6. PMID 9197246. S2CID 15594664.
- Rozovskaia T, Rozenblatt-Rosen O, Sedkov Y, Burakov D, Yano T, Nakamura T, et al. (January 2000). "Self-association of the SET domains of human ALL-1 and of Drosophila TRITHORAX and ASH1 proteins". Oncogene. 19 (3): 351–7. doi:10.1038/sj.onc.1203307. PMID 10656681. S2CID 43415797.
- Persson P, Jögi A, Grynfeld A, Påhlman S, Axelson H (July 2000). "HASH-1 and E2-2 are expressed in human neuroblastoma cells and form a functional complex". Biochemical and Biophysical Research Communications. 274 (1): 22–31. doi:10.1006/bbrc.2000.3090. PMID 10903890.
- Long RM, Gu W, Meng X, Gonsalvez G, Singer RH, Chartrand P (April 2001). "An exclusively nuclear RNA-binding protein affects asymmetric localization of ASH1 mRNA and Ash1p in yeast". The Journal of Cell Biology. 153 (2): 307–18. doi:10.1083/jcb.153.2.307. PMC 2169461. PMID 11309412.
- Parras CM, Schuurmans C, Scardigli R, Kim J, Anderson DJ, Guillemot F (February 2002). "Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity". Genes & Development. 16 (3): 324–38. doi:10.1101/gad.940902. PMC 155336. PMID 11825874.
- Sriuranpong V, Borges MW, Strock CL, Nakakura EK, Watkins DN, Blaumueller CM, et al. (May 2002). "Notch signaling induces rapid degradation of achaete-scute homolog 1". Molecular and Cellular Biology. 22 (9): 3129–39. doi:10.1128/MCB.22.9.3129-3139.2002. PMC 133746. PMID 11940670.
- Westerman BA, Neijenhuis S, Poutsma A, Steenbergen RD, Breuer RH, Egging M, et al. (April 2002). "Quantitative reverse transcription-polymerase chain reaction measurement of HASH1 (ASCL1), a marker for small cell lung carcinomas with neuroendocrine features". Clinical Cancer Research. 8 (4): 1082–6. PMID 11948117.
- Letinic K, Zoncu R, Rakic P (June 2002). "Origin of GABAergic neurons in the human neocortex". Nature. 417 (6889): 645–9. Bibcode:2002Natur.417..645L. doi:10.1038/nature00779. PMID 12050665. S2CID 4349070.
- de Pontual L, Népote V, Attié-Bitach T, Al Halabiah H, Trang H, Elghouzzi V, et al. (December 2003). "Noradrenergic neuronal development is impaired by mutation of the proneural HASH-1 gene in congenital central hypoventilation syndrome (Ondine's curse)". Human Molecular Genetics. 12 (23): 3173–80. doi:10.1093/hmg/ddg339. PMID 14532329.
- Sippel RS, Carpenter JE, Kunnimalaiyaan M, Chen H (December 2003). "The role of human achaete-scute homolog-1 in medullary thyroid cancer cells". Surgery. 134 (6): 866–71, discussion 871-3. doi:10.1016/s0039-6060(03)00418-5. PMID 14668716.
- Ferretti E, Di Stefano D, Zazzeroni F, Gallo R, Fratticci A, Carfagnini R, et al. (October 2003). "Human pituitary tumours express the bHLH transcription factors NeuroD1 and ASH1". Journal of Endocrinological Investigation. 26 (10): 957–65. doi:10.1007/bf03348192. PMID 14759067. S2CID 7358739.
- Mhawech P, Berczy M, Assaly M, Herrmann F, Bouzourene H, Allal AS, et al. (July 2004). "Human achaete-scute homologue (hASH1) mRNA level as a diagnostic marker to distinguish esthesioneuroblastoma from poorly differentiated tumors arising in the sinonasal tract". American Journal of Clinical Pathology. 122 (1): 100–5. doi:10.1309/QD0K-9Q1J-BH6B-5GQQ. PMID 15272537.
External links
- ASCL1+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human ASCL1 genome location and ASCL1 gene details page in the UCSC Genome Browser.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.