COVID-19 vaccination in India
On 16 January 2021, India started its national vaccination programme against the SARS-CoV-2 which is responsible for the COVID-19 pandemic. The drive prioritises healthcare and frontline workers, and then those over the age of 50 or suffering from certain medical conditions. According to health officials, India has administered 54,16,849 vaccine doses across the country as of 05 February 2021.[1]
| Part of a series on the |
| COVID-19 pandemic |
|---|
 |
|
|
|
Development
.jpg.webp)
Pune-based Serum Institute of India announced that it would apply for clinical trials of certain strains from Drug Controller General of India (DCGI) in April 2020. As per company president Adar Poonawalla, a vaccine for COVID-19 will be delivered within a year. However, it may not be effective on 20 to 30% people.[2] Two other companies are also trying to develop a vaccine: Zydus Cadila, which is replicating viral vector and developing a DNA plasmid vaccine,[3] and Hyderabad-based Bharat Biotech, in collaboration with US based FluGen, which is expecting the first clinical trials of a nasal vaccine by late 2020.[4] As of late February, the Serum Institute of India had begun animal trials of vaccine candidates,[5] followed by Zydus Cadila in March.[6] ICMR partnered with Bharat Biotech in May to develop COVID vaccine completely in India.[7] Till May, there were over 30 candidates of COVID-19 vaccine in development in India, many of which were already in pre-clinical tests.[8] Per reports emerged in July, ICMR was preparing to launch BBV152 COVID vaccine or Covaxin, India's first COVID-19 vaccine on 15 August following its ongoing human trials in July.[9] Although, later deadline was cited as only meant to cut "red tape" and expected timeline of any Indian vaccine not to be before 2021.[10] COVAXIN has been reported to have positive results on animals in building immunity against COVID-19 in pre-clinical trials.[11] In mid-July, Zydus Cadila too had followed with human trials of its vaccine named ZyCoV-D.[12] In early August, SII's got approval from DCGI for trial phases II & III.[13] SII has also joined GAVI in a partnership with Bill & Melinda Gates Foundation to produce 100 million doses of vaccine for developing countries.[14]
In September, India's science minister Dr. Harsh Vardhan announced that the first vaccine for use will be available by first quarter of 2021.[15] 30 million health workers directly dealing with COVID patients, especially doctors and other medical personnel are supposed to be first to receive the vaccine.[16]
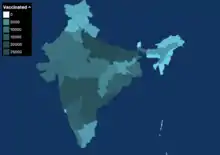
| Cumulative vaccination doses administered across the India, by date | |
1st doses 2nd doses | |
COVID-19 Vaccines with Approval for Emergency or Conditional Usage
.jpg.webp)
Covishield
On 1 January 2021, the Drug Controller General of India, approved the emergency or conditional use of AstraZeneca’s COVID-19 vaccine AZD1222 (marketed as Covishield).[17] Covishield is developed by the University of Oxford and its spin-out company, Vaccitech.[18] It’s a viral vector vaccine based on replication-deficient Adenovirus that causes cold in Chimpanzees. It can be stored, transported and handled at normal refrigerated conditions (two-eight degrees Celsius/ 36-46 degrees Fahrenheit). It has a shelf-life of at least six months.
Covaxin
On 2 January 2021, BBV152 (marketed as Covaxin), first indigenous vaccine, developed by Bharat Biotech in association with the Indian Council of Medical Research and National Institute of Virology received approval from the Drug Controller General of India for its emergency or conditional usage.[19] However, this approval was met with some concern as the vaccine had not then completed phase-3 trials.[20]
Vaccination drive
The national vaccination drive began on 16 January 2021, across 3,006 vaccination centres.[21]
Each vaccination centre will offer either Covishield or Covaxin, but not both. Some states have decided to use only Covishield as the “default option” and keep their Covaxin doses as a “buffer stock”.[20] Since Covaxin has not completed phase-3 trials, those receiving it will need to sign a signed consent form.[22]
On the first day (16 January), 1,65,714 people received the vaccination. There were some delays due to issues in uploading beneficiary lists at some sites.[23]
In the first three days, 6,31,417 people were vaccinated. Of these, 0.18% reported side-effects and nine people (0002%) were admitted to hospitals for observation and treatment.[24][25]
There is concern that the vaccine drive is being slowed down by low turnout, due to a combination of concerns about the safety of the vaccine, technical problems with the software used, and misinformation.[26]
Vaccine on order
| Vaccine | Progress | Doses ordered | Approval | Deployment |
|---|---|---|---|---|
| Oxford-AstraZeneca | Phase III clinical trials | 500 million[27] | ||
| Covaxin | *Phase III clinical trials in progress | 10 million | 01 January 2021(restricted)[30] | |
| Novavax | Phase III clinical trials | 1 billion[27] | Pending | Pending |
| Pfizer–BioNTech | Phase III clinical trials | Pending | Pending [lower-alpha 1] | Pending |
| Moderna | Phase III clinical trials | Pending | Pending | Pending |
| Sputnik V | Phase III clinical trials | 100 million[31] | Pending | Pending |
Vaccination by State
Andhra Pradesh
Andhra Pradesh had received 3,70,000 doses of Covishield and 20,000 of Covaxin. It was decided that only Covishield would be administered. The state aims to vaccinate 32,000 people a day. However, on the first two days, only 61 and 47.8 per cent of those days' targets could be vaccinated. There were two cases of adverse events, but neither required hospitalisation.[32]
Bihar
Bihar received 5,69,000 doses of the vaccine. 4,64,160 health workers had registered for the vaccine, and the state expects to vaccinate 30,000 people a day across 300 sites. Some doctors had doubts about the efficacy of Covaxin and refused to take it.[33]
Chattisgarh
One person was admitted to hospital following complications, but was later discharged.[24]
Delhi
4,319 health workers were administered the vaccine on the first day, and there were 51 minor and one major cases of adverse effects.[34] By day three, four people had been admitted to hospitals following complications, of which three had been later discharged.[24]
Karnataka
Karnataka Health Minister K. Sudhakar announced on 9 January that the state would be given 13.9 lakh (1.39 million) doses of Covishield in two batches. 6.3 lakh (630,000) health workers had registered for the vaccine.[35] Two people were admitted to hospital following complications; one person was later discharged.[24] A 43-year old vaccine recipient in Ballari died of a heart stroke two days after the jab.[36]
Kerala
Kerala initially received 4,33,500 doses of Covishield, and an additional batch of 3,60,500 Covishield doses were announced three days later. A total of 24,558 healthcare workers had been vaccinated on the first three days. No adverse reactions were reported. In total, 4,59,853 people from the state registered for the vaccine, including 1,75,673 healthcare workers from state-run hospitals, 1,99,937 from private hospitls, 2,932 from federal hospitals, 74,711 police staff and 6,600 municipal workers.[37]
Maharashtra
Maharashtra received 9,63,000 doses of Covishield and 20,000 doses of Covaxin. 7,85,000 health workers had registered to get the vaccination. 14,883 health workers in Maharashtra given COVID-19 vaccine on Tuesday[38] In Mumbai, only 1,926 people could be vaccinated on the first day, due to a combination of low turnout and technical problems with the software. The vaccination drive was temporarily suspended due to the technical glitches in the software alerting people to their vaccination appointments.[26]
Punjab
Health Minister Balbir Singh Sidhu announced around 1.60 lakh health workers in Punjab will receive a COVID-19 vaccine in the first phase, after that frontline worker and then people over the age of 50 or with co-morbid conditions. Vaccination inoculation of healthcare workers has been started in Punjab on 16-01-2021 at 59 designated sites across all the state.[39]
Phase 2
Vaccination of the frontline worker including police, local bodies, disaster management, rural development and panchayats and revenue departments will be starting from 1st of February 2021.[40]


| Order | Priority group | Number eligible (estimated) | Number of inoculated |
|---|---|---|---|
| 1 | Healthcare professionals, both government and private | 160,000 [41] | 6,8376 |
| 2 | Frontline worker including police, paramilitary forces, sanitation workers & disaster management volunteers | 300,000 [42] | 7,477 |
| 3 | People above 50 years and those aged below 50 with co-morbid conditions | TBA | |
| 4 | all those 18 years of age and over | TBA | |
| Total | 7,5853 | ||
| As of 2021-02-06 | |||
| Vaccine | Progress | Doses ordered for India | Doses allocated for Punjab | Approval | Deployment |
|---|---|---|---|---|---|
| Oxford-AstraZeneca | Phase III clinical trials | 500 million[43] | 204,500 | ||
| Covaxin | *Phase III clinical trials in progress | 10 million | Pending | 01 January 2021(restricted)[46] | ★16 January 2020[47] |
| Novavax | Phase III clinical trials | 1 billion[48] | Pending | Pending | Pending |
| Moderna | Phase III clinical trials | Pending | Pending | Pending | Pending |
| Sputnik V | Phase III clinical trials | 100 million[49] | Pending | Pending | Pending |
★ Not deployed in Punjab yet | |||||
Rajasthan
One person was admitted to Bangar District Hospital following suspected anaphylaxis.[24]
Tamil Nadu
Tamil Nadu received 5,36,000 doses of Covishield and 20,000 doses of Covaxin.[50]
Uttarkhand
One person was admitted to hospital following complications, but was later discharged.[24]
References
- "COVID19 INDIA".
- "Coronavirus vaccine within a year but it won't be 100% effective". The Economic Times. 21 March 2020. Retrieved 22 March 2020.
- Jayakumar, PB (5 April 2020). "Zydus Cadila, Serum Institute too in the hunt for coronavirus vaccine". India Today. Retrieved 6 April 2020.
- "Hyderabad-based biotech firm working on nasal vaccine for Covid-19". India Today. 3 April 2020. Retrieved 6 April 2020.
- Khelkar, Pankaj P. (19 February 2020). "Indian company first to test coronavirus vaccine on animals, human trials expected in 6 months". India Today. Retrieved 13 April 2020.
- "The experiment of coronavirus vaccine on animals started in India, hopefully desired results will come in 4–6 months". Inventia. 8 April 2020. Retrieved 13 April 2020.
- Chakrabarti, Angana (10 May 2020). "India to develop 'fully indigenous' Covid vaccine as ICMR partners with Bharat Biotech". The Print. Retrieved 11 May 2020.
- Ray, Meenakshi (6 May 2020). "30 Covid-19 vaccines in different stages of development: Scientists to PM Modi". Hindustan Times. Retrieved 10 May 2020.
- Milan Sharma (3 July 2020). "Bharat Biotech-ICMR to launch indigenous Covid vaccine by August 15". India Today. Retrieved 3 July 2020.
- Mathur, Swati (11 July 2020). "Covid-19 vaccine unlikely before 2021, House panel told". The Times of India. Retrieved 16 July 2020.
- "India's coronavirus vaccine candidate COVAXIN showed positive result in animals: Bharat Biotech". Daily News & Analysis. 12 September 2020. Retrieved 13 September 2020.
- "Coronavirus vaccine update: India's second COVID-19 vaccine candidate 'ZyCoV-D' to start human trials; here is all you need to know". The Times of India. 15 July 2020. Retrieved 16 July 2020.
- "Coronavirus vaccine: DCGI gives nod to Serum-Oxford for phase 2, 3 clinical trials in India". Daily News & Analysis. 3 August 2020. Retrieved 4 August 2020.
- "Serum Institute to produce up to 100 million Covid-19 vaccine doses for India, other countries". The Times of India. 7 August 2020. Retrieved 10 August 2020.
- "Expect Covid-19 vaccine by early next year, will take first shot if any trust deficit: Vardhan". The Times of India. 13 September 2020. Retrieved 13 September 2020.
- Kaul, Rhythma (21 October 2020). "30 Million Frontline Workers To Get Covid-19 Vaccine In Phase 1". Hindustan Times. New Delhi. Retrieved 21 October 2020.
- "COVID-19 vaccine Covishield gets approval from DCGI's expert panel". The Hindu. 1 January 2021. Retrieved 2 January 2021.
- "AstraZeneca's COVID-19 vaccine authorised for emergency supply in the UK". AstraZeneca. AstraZeneca. 30 December 2020. Retrieved 2 January 2021.
- "Expert panel recommends Bharat Biotech's Covaxin for restricted emergency use". News18. 2 January 2021. Retrieved 2 January 2021.
- Prasad, R (15 January 2020). "Vaccine dilemma — to take or not to take Covaxin". The Hindu. Chennai. Retrieved 16 January 2020.
- "World's largest vaccination programme begins in India on January 16". The Hindu. 15 January 2021. Retrieved 16 January 2021.
- Bindu Shajan Perappadan (16 January 2020). "Covaxin recipients asked to sign consent form on 'clinical trial mode'". The Hindu. New Delhi. Retrieved 16 January 2020.
- "No case of post-vaccination hospitalisation reported so far: Health Ministry". The Hindu. New Delhi. 16 January 2020. Retrieved 16 January 2020.
- "Over six lakh vaccinated so far". The Hindu. New Delhi. 19 January 2020. Retrieved 19 January 2020.
- "കോവിഡ് വാക്സിനേഷന്: പാര്ശ്വഫലമുണ്ടായത് 0.18% പേരില്, ആശുപത്രിയില് പ്രവേശിപ്പിച്ചത് 0.002% പേരെ". Mathrubhumi. New Delhi. 19 January 2020. Retrieved 19 January 2020.
- Hannah Ellis-Peterson; Amrit Dhillon (20 January 2020). "Indian hesitancy sets back world's biggest Covid vaccination drive". The Guardian. New Delhi. Retrieved 20 January 2020.
- "Corona vaccine update: At 1.6 billion doses, India No. 1 in deals for Covid vaccine: Study | India News - Times of India". The Times of India.
- "Oxford Covid vaccine approved, three more awaiting nod, confirms Javadekar | India News - Times of India". The Times of India.
- Perappadan, Bindu Shajan (9 January 2021). "Coronavirus | First phase of vaccination to start on January 16" – via www.thehindu.com.
- "India's Wait Over, Drug Regulator Says Covid Vaccines Cleared "110% Safe"". NDTV.com.
- Kumar, Chethan (4 December 2020). "Corona vaccine update: At 1.6 billion doses, India No. 1 in deals for Covid vaccine: Study". The Times of India. Retrieved 28 January 2021.
- "13,041 persons turn up to get the jab on Day 2". The Hindu. Vijayawada. 17 January 2020. Retrieved 19 January 2020.
- Manoj, CK (16 January 2020). "COVID-19 vaccination begins in Bihar; 30,000 health workers to be inoculated today". Down To Earth. Patna. Retrieved 19 January 2020.
- "One 'severe', 51 'minor' cases of post-vaccination adverse events reported among health workers in Delhi". The Hindu. New Delhi. 17 January 2020. Retrieved 19 January 2020.
- "Karnataka will get 13.9 lakh vials of COVID-19 vaccine soon: Sudhakar". The Hindu. 9 January 2021. Retrieved 19 January 2021.
- "Karnataka's Covid vaccine recipient dies of heart stroke: Minister". Business Insider. 18 January 2021. Retrieved 19 January 2021.
- "സംസ്ഥാനത്തേക്ക് 3,60,500 ഡോസ് കോവിഡ് വാക്സിന് കൂടി; ഇതുവരെ വാക്സിന് സ്വീകരിച്ചത് 24,558 പേർ". Mathrubhumi. Thiruvananthapuram. 19 January 2020. Retrieved 19 January 2020.
- "14,883 health workers in Maharashtra given Covid-19 vaccine on 16 JAN". The Hindu Business Line. Mumbai. 19 January 2020. Retrieved 16 January 2020.
- https://www.tribuneindia.com/news/punjab/vaccination-at-59-sites-5-900-to-get-jab-today-in-punjab-199060
- https://www.tribuneindia.com/news/punjab/after-health-staff-others-to-get-jabs-from-tomorrow-205628
- https://www.business-standard.com/article-amp/current-affairs/160-000-health-workers-to-be-first-to-get-covid-vaccine-in-punjab-minister-121010300391_1.html
- https://www.livemint.com/politics/policy/160-lakh-health-workers-will-be-first-to-get-covid-19-vaccine-in-punjab-11609698076145.html
- https://timesofindia.indiatimes.com/india/at-1-6-billion-doses-india-no-1-in-deals-for-covid-vaccine-study/articleshow/79555922.cms
- https://timesofindia.indiatimes.com/india/oxford-covid-vaccine-approved-three-more-awaiting-nod-confirms-javadekar/articleshow/80073961.cms
- https://www.thehindu.com/news/national/india-to-start-covid-19-vaccination-drive-on-jan-16/article33536670.ece
- https://www.ndtv.com/india-news/oxford-covid-19-vaccine-bharat-biotechs-covaxin-get-final-approval-by-drug-regulator-will-be-indias-first-vaccines-2347053
- https://www.thehindu.com/news/national/india-to-start-covid-19-vaccination-drive-on-jan-16/article33536670.ece
- https://timesofindia.indiatimes.com/india/at-1-6-billion-doses-india-no-1-in-deals-for-covid-vaccine-study/articleshow/79555922.cms
- https://timesofindia.indiatimes.com/india/at-1-6-billion-doses-india-no-1-in-deals-for-covid-vaccine-study/articleshow/79555922.cms
- "Tamil Nadu gears up for Covid vaccine rollout". The Hindu Business Line. Chennai. 12 January 2020. Retrieved 16 January 2020.
Footnotes
- Pfizer applied for Emergency Use Approval (EUA) to the Drugs Controller General of India (DCGI), but did not appear to present its case to the Expert Committee despite three opportunities
External links
- "Coronavirus Vaccine Tracker". The New York Times.
- COVID-19 vaccine tracker, Regulatory Focus
- "STAT's Covid-19 Drugs and Vaccines Tracker". Stat.
- Levine, Hallie (23 September 2020). "The 5 Stages of COVID-19 Vaccine Development: What You Need to Know About How a Clinical Trial Works". Johnson & Johnson.
- "COVID-19 vaccines: development, evaluation, approval and monitoring". European Medicines Agency.
- COVID-19 vaccination in India tracker with historical data per day